Abstract
Background and objectives
Sleep duration has been associated with cardiometabolic risk and mortality. The health-related quality of life represents a patient’s comprehensive perception of health and is accepted as a health outcome. We examined the relationship between sleep duration and health-related quality of life in predialysis CKD.
Design, setting, participants, & measurements
In this cross-sectional study, data from 1910 adults with CKD enrolled in the Korean Cohort Study for Outcome in Patients with CKD were analyzed. Health-related quality of life was assessed with the physical component summary and mental component summary of the Short Form-36 Health Survey. Low health-related quality of life was defined as a Short Form-36 Health Survey score >1 SD below the mean. Using a generalized additive model and multivariable logistic regression analysis, the relationship between self-reported sleep duration and health-related quality of life was examined.
Results
Seven-hour sleepers showed the highest health-related quality of life. We found an inverted U-shaped relationship between sleep duration and health-related quality of life as analyzed by a generalized additive model. In multivariable logistic analysis, short sleepers (≤5 h/d) had lower health-related quality of life (odds ratio, 3.23; 95% confidence interval, 1.86 to 5.60 for the physical component summary; odds ratio, 2.37; 95% confidence interval, 1.43 to 3.94 for the mental component summary), and long sleepers (≥9 h/d) had lower health-related quality of life (odds ratio, 2.80; 95% confidence interval, 1.55 to 5.03 for the physical component summary; odds ratio, 2.08; 95% confidence interval, 1.20 to 3.60 for the mental component summary) compared with 7-hour sleepers. Sleep duration had a significant U-shaped association with low health-related quality of life.
Conclusions
These findings suggest that short or long sleep duration is independently associated with low health-related quality of life in adults with CKD.
Keywords: Adult; chronic kidney disease; Cohort Studies; Cross-sectional Studies; Humans; Logistic Models; Perception; quality of life; Renal Insufficiency, Chronic; Self Report; Sleep; Sleep Duration; Time Factors
Introduction
CKD is a common health problem with high health and economic burdens (1). Sleep occupies between 20% and 40% of the day and is essential for human health (2). Sleep duration has declined and sleep problems have increased in current society (3,4). Adequate sleep is important to restore functional capacity and maintain homeostasis in the kidney (5,6). The influences of sleep on kidney disease are complex and little known.
There is growing evidence that sleep is related to health outcome. Recent studies have suggested that insomnia is associated with higher risk of developing and dying from cardiovascular disease (7). Short or long sleep duration is associated with higher mortality compared with 7 hours of sleep duration (8). Sleep disorders, such as insomnia, restless leg syndrome, and sleep apnea, are prevalent in patients with CKD, in particular those undergoing dialysis (9). Nearly one half of patients on hemodialysis were reported to experience poor sleep quality, which was associated with higher mortality (10). However, few studies have attempted to characterize sleep duration in predialysis CKD. Self-reported sleep duration <5 hours was associated with faster decline in kidney function compared with sleeping 7–8 hours in healthy women (11). Short sleep duration measured by actigraphy was a risk factor for CKD progression in individuals with CKD (12).
The heath-related quality of life (HRQOL) represents a patient’s comprehensive perception of physical and mental health, and it is accepted as a health outcome. Patients with advanced CKD have multiple, complex comorbid conditions that can produce poor HRQOL (13). Better understanding of HRQOL enables medical providers to deliver patient-centered care and improve the wellbeing of patients, but HRQOL remains an underinvestigated issue in CKD (14). Fatigue, lack of energy, and drowsiness are the most common symptoms that result in the poor HRQOL in predialysis CKD (15). Sleep disorders and short sleep have emerged as factors related to adverse cardiometabolic risk and mortality. However, the relation of sleep duration with HRQOL in predialysis CKD has not been shown.
The Korean Cohort Study for Outcome in Patients with CKD (KNOW-CKD) (16) is a cohort study in adults with all stages of CKD. The primary aim of the KNOW-CKD is to identify risk factors related to CKD and its outcomes. To improve our understanding of sleep and HRQOL in adults with predialysis CKD, we analyzed the association between sleep duration and HRQOL in the cross-sectional baseline data of the KNOW-CKD.
Materials and Methods
Participants
The KNOW-CKD is an ongoing multicenter, prospective, observational study of adults with CKD in Korea. The study design has been published previously (16) and is summarized here. Nine clinical centers in university-affiliated hospitals enrolled 2238 adults with CKD from 2011 to 2016. We defined CKD as eGFR<60 ml/min per 1.73 m2, albuminuria, or structural abnormalities for >3 months (17), and we excluded subjects with previous dialysis, organ transplantation, advanced heart failure, liver cirrhosis, history of malignancy, current pregnancy, or single kidney and those unable to give written consent. The causes of CKD were diabetes, hypertension, GN, and autosomal dominant polycystic kidney disease (16). The KNOW-CKD enrolled ethnic Koreans who ranged in age between 20 and 75 years old and had eGFR ranging from 6 to 145 ml/min per 1.73 m2. We analyzed 1910 participants from this cohort who underwent complete baseline laboratory tests and completed questionnaires of sleep duration and HRQOL (Supplemental Figure 1). This was a cross-sectional study designed to assess the associations of sleep duration with HRQOL in adults with CKD.
Variables
The enrolled adults with CKD were screened and evaluated at baseline for sociodemographic information, lifestyle information, detailed medical histories, anthropometric measurement, and laboratory tests. Variables for this study included both survey data and laboratory data: age, sex, marriage, household income, education level, smoking, alcohol drinking, physical activity, diabetes mellitus, hypertension, cardiovascular disease, serum hemoglobin, albumin, creatinine, and 24-hour urine protein. Low income status was defined as a monthly family income less than approximately United States $1500 US. Low education level was defined as an academic background of less than high school graduation. Hypertension was defined as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or a previous diagnosis. Diabetic mellitus was defined as serum hemoglobin A1c ≥6.5%, fasting glucose ≥126 mg/dl, or a previous diagnosis of diabetes. Alcohol drinking was defined as having more than two drinks a day in the past month. Health-enhancing physical activity was defined as >150 min/wk mode rate activity, 75 min/wk vigorous activity, or an equivalent combination according to global recommendations of the World Health Organization. Cardiovascular disease was defined as a medical history of myocardial infarction, heart failure, peripheral vascular disease, or stroke.
Serum creatinine level was measured by the isotope dilution mass spectroscopy traceable method. The eGFR was calculated using the four-variable Chronic Kidney Disease Epidemiology Collaboration equation (17). The stage of CKD was on the basis of GFR category of the Kidney Disease Improving Global Outcomes 2012 Clinical Practice Guideline for the Evaluation and Management of CKD (17).
Sleep Duration
Sleep duration was assessed by the following question: “During the past month, how many hours of actual sleep do you get a day? This can be different than the number of hours you spend in bed.” The participants recalled and self-reported the total sleep duration per day for the last month on average. Sleep duration was rounded to the nearest hour and categorized as ≤5, 6, 7, 8, or ≥9 hours. Short sleepers were defined as adults who sleep ≤5 h/d, and long sleepers were defined as adults who sleep ≥9 h/d.
HRQOL
The Kidney Disease Quality of Life instrument (KDQOL-SF) was used to assess comprehensive HRQOL in patients with kidney disease (18). The KDQOL-SF includes the Medical Outcome Study Short Form-36 Health Survey (SF-36), and it is supplemented with kidney disease–targeted items. The Korean versions of KDQOL-SF (19) and SF-36 (20) used in this study have been verified in previous studies and include eight subscales: physical function, role of physical limitation due to physical problems, bodily pain, general health, vitality, role of emotional limitation due to emotional problems, social function, and mental health. KDQOL-SF and Health Questionnaires were completed with the aid of study personnel if adults with CKD had difficulty with self-administration.
Responses to each question were transformed into SF-36 equivalent scores, and each scale ranges from 0 to 100, with higher numerical scores indicating better HRQOL or less impairment in that subscale. The four subscales of physical function, role of physical limitation due to physical problems, bodily pain, and general health are summarized in a physical component summary (PCS), and the four subscales of vitality, role of emotional limitation due to emotional problems, social function, and mental health are summarized in a mental component summary (MCS). In this study, PCS and MCS scores were the outcomes of interest. Low HRQOL (low PCS or low MCS) was defined as SF-36 score >1 SD below the mean.
Statistical Methods
Data are expressed as a percentile for categorical variables and mean±SD or median (interquartile range) for continuous variables. Chi-squared analysis for categorical variables and ANOVA for continuous variables were used to determine differences in baseline characteristics and HRQOL parameters according to eGFR stage and sleep duration. Tests for trend were performed using mean values in the categories as continuous variables in the logistic regression models. Because many parameters might be inter-related with HRQOL, we constructed multivariable linear regression models to determine independent associations between sleep duration and HRQOL and reported the results as regression coefficient (β) and 95% confidence interval (95% CI). Multiple parameters were selected on the basis of our baseline data and other HRQOL studies of CKD. Because sleep duration of 7 h/d showed the highest HRQOL in this study and was recommended as an adequate sleep duration to promote optimal health (21), we analyzed the relationship between sleep duration and HRQOL in reference to adults who slept 7 h/d. Multivariable linear regression analysis was used to analyze the shape of the association between sleep duration and HRQOL after adjusting for potential confounding variables, such as socioeconomic factors, health-related behavior, comorbidities, and laboratory findings. Model 1 was adjusted for age, sex, eGFR, body mass index, hemoglobin, serum albumin diabetes, hypertension, and cardiovascular disease. Model 2 was adjusted for model 1 variables plus 24-hour urine protein, alcohol drinking, smoking, health-enhancing physical activity, marriage, low education, and low income. Because a generalized additive model provides a flexible and effective technique for modeling nonlinear data, we used a generalized additive model to shape margin plots of the association between sleep duration and HRQOL. Marginal effect was estimated by margin command and plotted by the marginsplot command in Stata software adjusted for the predictors in model 2. Multivariable logistic regression analysis was used to analyze the odds ratios (ORs) for poor HRQOL. All statistical analyses were performed using Stata Version 14 (StataCorp LP, College Station, TX).
Results
Participants and Characteristics
A total of 1910 adults with predialysis CKD completed the SF-36 and the sleep questionnaire and underwent tests for variables of interest at the baseline study of the KNOW-CKD. The overall mean age was 52±12 years old, and 62% of participants were men. The mean eGFR was 53±30 ml/min per 1.73 m2, and the median 24-hour urine protein was 546 mg/d. Diabetes was present in 33% of participants, and cardiovascular disease was present in 11% of participants. Adults in advanced CKD stage had low socioeconomic status, multiple comorbidities, lower hemoglobin, and lower albumin level. PCS and MCS were lower with advanced CKD stage. The overall sleep duration was 6.9±1.3 hours. Short sleepers accounted for 11% of participants, and long sleepers for accounted for 7% of participants. The proportion of long sleepers was higher in lower eGFR than higher eGFR (P for trend <0.001), but that of short sleepers was not different (P for trend =0.66) (Supplemental Table 1).
Sleep Duration and HRQOL
Table 1 presents the characteristics of the study subjects according to sleep duration. Adults with long sleep duration had lower hemoglobin, lower albumin level, lower eGFR, more prevalent diabetes, and more severe proteinuria. Seven-hour sleepers showed the highest scores on the SF-36 (PCS, 76±16 and MCS, 72±16) and had the lowest prevalence of low HRQOL (9% of low PCS and 13% of low MCS). Short sleepers (≤5 hours) and long sleepers (≥9 hours) were more prevalent in the adults with low HRQOL (27% and 32% of low PCS, respectively, and 25% and 30% of low MCS, respectively) compared with 7-hour sleepers. Adults with long sleep duration showed lower PCS score than those with short sleep duration (P for trend <0.01).
Table 1.
Baseline characteristics of 1910 adults with CKD on the basis of category of sleep duration
| Characteristic | Missing Data, % | Sleep Duration, h | ||||
|---|---|---|---|---|---|---|
| ≤5, n=204 | 6, n=498 | 7, n=567 | 8, n=504 | ≥9, n=137 | ||
| Age, yr | 0 | 55±12 | 53±12 | 53±12 | 53±12 | 55±13 |
| Men, % | 0 | 52 | 65 | 64 | 62 | 59 |
| eGFR, ml/min per 1.73 m2a | 0 | 54±30 | 56±32 | 55±31 | 52±31 | 39±26 |
| 24-h Urine protein, mg/db | 86 (5) | 383 [130–1190] | 501 [154–1313] | 600 [178–1551] | 600 [176–1781] | 852 [286–2440] |
| Alcohol drinking, % | 0 | 30 | 37 | 39 | 34 | 34 |
| Active smoking, % | 0 | 14 | 15 | 17 | 18 | 17 |
| Health-enhancing physical activity, % | 78 (4) | 38 | 43 | 44 | 43 | 35 |
| Body mass index, kg/m2 | 0 | 24.7±3.4 | 24.6±3.3 | 24.2±3.3 | 24.4±3.4 | 25.1±3.5 |
| Hemoglobin, g/dla | 0 | 12.9±1.9 | 13.1±1.9 | 13.0±2.1 | 12.7±2.0 | 11.9±2.0 |
| Serum albumin, g/dla | 0 | 4.2±0.4 | 4.2±0.5 | 4.2±0.4 | 4.2±0.4 | 4.0±0.5 |
| Unmarried, % | 46 (2) | 18 | 17 | 15 | 16 | 18 |
| Low income, % | 27 (1) | 34 | 19 | 18 | 25 | 39 |
| Low education, % | 0 | 29 | 20 | 20 | 26 | 31 |
| Diabetes, %a | 0 | 29 | 30 | 30 | 35 | 48 |
| Hypertension, %b | 0 | 92 | 96 | 96 | 97 | 99 |
| Cardiovascular disease, % | 0 | 7 | 12 | 9 | 12 | 18 |
| Physical component summaryb | 0 | 67±21 | 75±17 | 76±16 | 71±18 | 62±21 |
| Mental component summary | 0 | 64±21 | 72±17 | 72±16 | 69±18 | 62±20 |
| Low physical component summary, % | 0 | 27 | 12 | 9 | 18 | 32 |
| Low mental component summary, % | 0 | 25 | 11 | 13 | 16 | 30 |
Data are expressed as mean±SD or median [interquartile range].
P for trend <0.001.
P for trend <0.01.
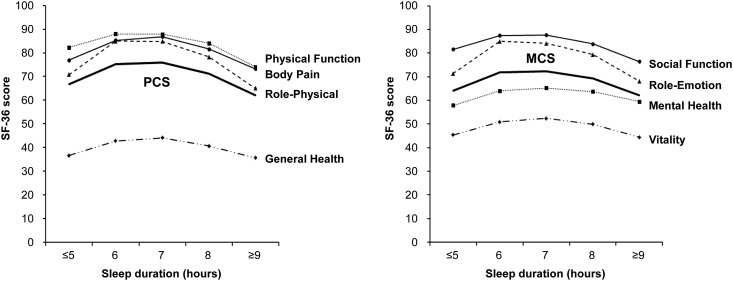
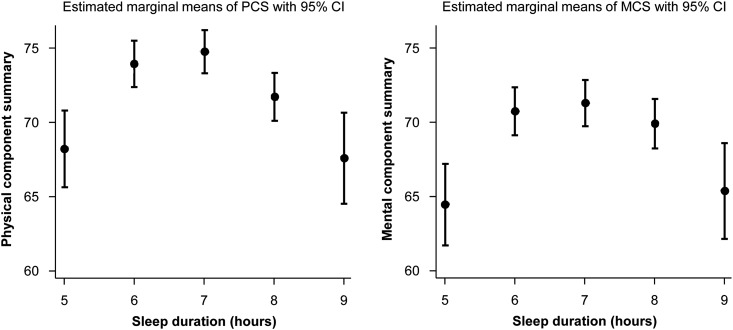
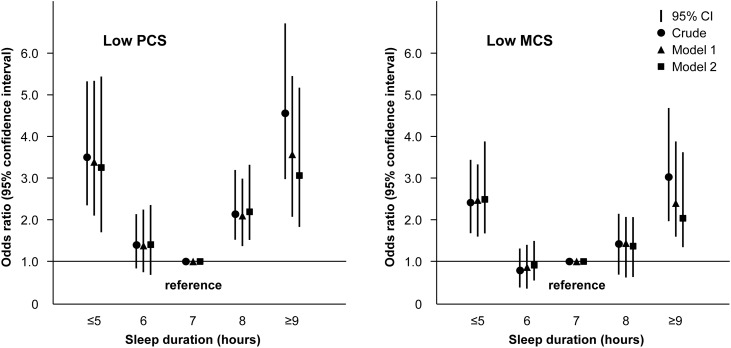
Sleep duration showed an inverted U-shaped relationship with SF-36 score and all subscale scores, and 7-hour sleep duration was associated with the highest HRQOL (Figure 1). In the relationships of sleep duration with PCS and MCS using a generalized additive model, inverted U-shaped associations were observed (Figure 2). When we explored the nonlinear relationships of sleep duration with HRQOL by multivariable linear regression model, short sleepers had lower HRQOL (β=−6.54; 95% CI, −9.56 to −2.94 for PCS; β=−6.81; 95% CI, −9.98 to −3.64 for MCS) and long sleepers had lower HRQOL (β=−7.10; 95% CI, −10.53 to −13.67 for PCS; β=−5.86; 95% CI, −9.47 to −2.26 for MCS) compared with 7-hour sleepers (Table 2). In the multivariable logistic regression model, the short sleepers had higher ORs for low HRQOL (OR, 3.23; 95% CI, 1.86 to 5.60 for PCS; OR, 2.37; 95% CI, 1.43 to 3.94 for MCS) and the long sleepers had higher ORs for low HRQOL (OR, 2.80; 95% CI, 1.55 to 5.03 for PCS; OR, 2.08; 95% CI, 1.20 to 3.60 for MCS) compared with 7-hour sleepers. A U-shaped relationship was observed between sleep duration and low HRQOL (Figure 3).
Figure 1.
Distribution of the physical component score (PCS) and the mental component score (MCS) of the Short Form-36 Health Survey (SF-36) according to sleep duration in the 1910 adults with CKD.
Figure 2.
Relationship of sleep duration with the physical component score (PCS) and the mental component score (MCS) compared with referent 7-hour sleep duration predicted by multivariable regression model. Marginal effect was estimated by Stata software adjusted to the predictors in model 2. 95% CI, 95% confidence interval.
Table 2.
Linear regression model for health-related quality of life by sleep duration in 1910 adults with predialysis CKD
| Sleep duration | Univariable | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| βa | 95% CI | βa | 95% CI | βa | 95% CI | |
| Physical component summary | ||||||
| Sleep duration, h | ||||||
| ≤5 | −9.22 | −12.09 to −6.35; P<0.001 | −7.96 | −10.65 to −5.27; P<0.001 | −6.54 | −9.56 to −2.94; P<0.001 |
| 6 | −0.62 | −2.78 to 1.54; P=0.57 | −0.60 | −2.60 to 1.40; P=0.56 | −0.78 | −2.94 to 1.39; P=0.48 |
| 7 | 0.00 | Reference | 0.00 | Reference | 0.00 | Reference |
| 8 | −4.75 | −6.90 to −2.60; P<0.001 | −3.59 | −5.59 to −1.59; P<0.001 | −2.97 | −5.15 to −0.80; P<0.01 |
| ≥9 | −13.88 | −17.23 to −10.53; P<0.001 | −9.58 | −12.73 to −6.44; P<0.001 | −7.10 | −10.53 to −3.67; P<0.001 |
| R2 | 0.05 | 0.21 | 0.29 | |||
| Mental component summary | ||||||
| Sleep duration, h | ||||||
| ≤5 | −8.25 | −11.13 to −5.38; P<0.001 | −7.95 | −10.79 to −5.12; P<0.001 | −6.81 | −9.98 to −3.64; P<0.001 |
| 6 | −0.51 | −2.68 to 1.65; P=0.64 | −0.78 | −2.89 to 1.33; P=0.47 | −0.52 | −2.79 to 1.76; P=0.66 |
| 7 | 0.00 | Reference | 0.00 | Reference | 0.00 | Reference |
| 8 | −3.13 | −5.29 to −0.98; P=0.004 | −2.53 | −4.64 to −0.42; P=0.02 | −1.34 | −3.62 to 0.94; P=0.25 |
| ≥9 | −10.24 | −13.59 to −6.88; P<0.001 | −7.80 | −11.1 to −4.48; P<0.001 | −5.86 | −9.47 to −2.26; P<0.001 |
| R2 | 0.03 | 0.10 | 0.19 | |||
Model 1 (n=1910) is adjusted for age, sex, eGFR, body mass index, hemoglobin, serum albumin, diabetes, hypertension, and cardiovascular disease. Model 2 (n=1748) is adjusted for model 1 variables plus 24-hour urine protein, alcohol drinking, smoking, health-enhancing physical activity, marriage, low education, and low income. 95% CI, 95% confidence interval.
β is estimated using regression coefficients from a linear regression model.
Figure 3.
Sleep duration categories show a U-shaped association with health-related quality of life. Logistic regression was adjusted for age, sex, eGFR, body mass index, hemoglobin, serum albumin, diabetes, hypertension, and cardiovascular disease in model 1 and model 1 variables plus 24-hour urine protein, alcohol drinking, smoking, health-enhancing physical activity, marriage, low education, and low income in model 2.
Subgroup Analyses
The association was not modified in selected subgroups of age, sex, diabetes, eGFR, and cardiovascular disease. The association between sleep duration and low HRQOL also showed a U-shaped pattern in subgroup analysis (Table 3).
Table 3.
Multivariable logistic regression models for low health-related quality of life by sleep duration in selected subgroups
| Group | Sleep duration, h | ||||
|---|---|---|---|---|---|
| ≤5 | 6 | 7 | 8 | ≥9 | |
| Low physical component summary | |||||
| Age, yr | P for interaction =0.52 | ||||
| <65, n=1520 | 3.14 (1.65–5.98) | 1.13 (0.64–2.00) | Reference | 1.67 (1.00–2.80) | 2.30 (1.10–4.77) |
| ≥65, n=390 | 4.64 (1.49–14.47) | 2.56 (0.94–6.91) | Reference | 4.17 (1.56–11.16) | 5.44 (1.79–16.52) |
| Sex | P for interaction =0.67 | ||||
| Men, n=1179 | 3.59 (1.59–8.13) | 1.13 (0.57–2.26) | Reference | 1.55 (0.82–2.95) | 2.56 (1.12–5.81) |
| Women, n=731 | 3.30 (1.53–7.11) | 1.65 (0.83–3.31) | Reference | 2.98 (1.56–5.70) | 3.28 (1.39–7.72) |
| Diabetes | P for interaction =0.34 | ||||
| Yes, n=621 | 2.84 (1.26–6.38) | 1.05 (0.55–1.99) | Reference | 1.26 (0.69–2.30) | 2.10 (0.97–4.53) |
| No, n=1298 | 4.25 (1.84–9.80) | 1.20 (0.91–4.31) | Reference | 3.53 (1.71–7.30) | 4.93 (1.88–12.92) |
| eGFR, ml/min per 1.73 m2 | P for interaction =0.15 | ||||
| <60, n=1237 | 3.67 (1.96–6.87) | 1.30 (0.76–2.23) | Reference | 1.64 (0.99–2.96) | 2.32 (1.23–4.37) |
| ≥60, n=673 | 4.06 (0.93–17.75) | 2.24 (0.62–8.07) | Reference | 6.35 (1.87–21.58) | 12.44 (2.17–71.23) |
| Cardiovascular disease | P for interaction =0.50 | ||||
| Yes, n=206 | 6.98 (1.13–43.03) | 1.20 (0.30–4.79) | Reference | 1.14 (0.28–4.58) | 3.45 (0.71–16.70) |
| No, n=1704 | 2.89 (1.60–5.20) | 1.37 (0.81–2.30) | Reference | 2.14 (1.33–3.45) | 2.69 (1.41–5.12) |
| Low mental component summary | |||||
| Age, yr | P for interaction =0.15 | ||||
| <65, n=1520 | 3.21 (1.79–5.73) | 0.87 (0.52–1.46) | Reference | 1.06 (0.66–1.72) | 1.88 (0.94–3.75) |
| ≥65, n=390 | 1.36 (0.43–4.26) | 1.28 (0.49–3.38) | Reference | 2.24 (0.87–5.75) | 3.44 (1.18–10.02) |
| Sex | P for interaction =0.92 | ||||
| Men, n=1179 | 2.64 (1.26–5.55) | 0.96 (0.52–1.77) | Reference | 1.29 (0.73–2.28) | 2.55 (1.22–5.35) |
| Women, n=731 | 2.10 (1.01–4.39) | 0.80 (0.46–1.77) | Reference | 1.29 (0.69–2.43) | 1.73 (0.73–4.11) |
| Diabetes | P for interaction =0.10 | ||||
| Yes, n=621 | 1.49 (0.66–3.41) | 1.12 (0.60–2.10) | Reference | 0.80 (0.43–1.48) | 1.96 (0.93–4.16) |
| No, n=1298 | 3.40 (1.74–6.62) | 0.78 (0.40–1.50) | Reference | 1.66 (0.94–2.95) | 2.33 (1.00–5.45) |
| eGFR, ml/min per 1.73 m2 | P for interaction =0.28 | ||||
| <60, n=1237 | 2.59 (1.44–4.64) | 0.97 (0.58–1.62) | Reference | 1.07 (0.67–1.73) | 1.76 (0.96–3.22) |
| ≥60, n=673 | 2.21 (0.71–6.93) | 0.98 (0.37–2.59) | Reference | 2.28 (0.92–5.64) | 9.15 (2.26–37.09) |
| Cardiovascular disease | P for interaction =0.37 | ||||
| Yes, n=206 | 7.01 (0.81–60.46) | 1.84 (0.35–9.59) | Reference | 1.64 (0.30–8.94) | 6.00 (0.98–36.88) |
| No, n=1704 | 2.09 (1.22–3.57) | 0.87 (0.54–1.41) | Reference | 1.18 (0.77–1.82) | 1.72 (0.93–3.18) |
Values are expressed as odds ratio (95% confidence interval). Multivariable logistic regression is adjusted in model 2.
Discussion
In this cross-sectional study of adults with predialysis CKD, HRQOL was lower and sleep duration was longer with advanced CKD stage. Sleep duration had a U-shaped association with low HRQOL, and 7-h/d sleep duration showed the highest HRQOL. Short and long sleep durations have an association with low HRQOL in predialysis CKD.
Sleep is well documented as an important contributor to health, and it is regulated by neurohormonal factors and sociocultural factors (2). There is growing evidence that sleep duration and sleep disorders are related to cardiometabolic diseases, such as obesity (22), hypertension (23), type 2 diabetes mellitus (24), and cardiovascular disease (25). A meta-analysis study (8) showed a J-shaped association between sleep duration and all-cause mortality, and both shortened and prolonged sleep durations were associated with greater mortality compared with 7-hour sleep duration in the general population. Sleep disorders, such as insomnia, restless leg syndrome, periodic limb movements, and sleep apnea, are common in patients with CKD (5,9,26), in particular those with ESKD (27). Kidney physiology is modulated by the sleep-wake cycle, including molecular expression of nephrons (28), sodium reabsorption (29), and glomerular filtration (30). Thus, disturbance of sleep and kidney function can have adverse effects on each other. In a cross-sectional study of Chinese adults, worse sleep quality was associated with high risk for CKD and proteinuria (31). In an analysis of the Nurses’ Health Study, self-reported sleep duration <5 hours was associated with faster decline in kidney function compared with sleeping 7–8 hours in healthy women (11). In the Chronic Renal Insufficiency Cohort Study, poor-quality sleep and short sleep duration measured by actigraphy were risk factors for CKD progression in individuals with CKD (12). However, there is little known about the relationship between sleep duration and HRQOL in predialysis CKD.
HRQOL is a patient-centered outcome on the basis of a patient’s perception of physical, mental, and social wellbeing that is reflected by the World Health Organization’s definition of health. Improving HRQOL is a key health goal. Many individuals with predialysis CKD are often unaware of their disease before it becomes advanced CKD and have nonspecific symptoms, such as fatigue or lack of energy (15). It is uncertain if these symptoms are produced by anemia, malnutrition, comorbidities, or the socioeconomic environment rather than decreased kidney function itself. Poor HRQOL and its progression to poor outcome have been well documented in patients on dialysis (13,32), but the causes and consequences in predialysis CKD have been a challenge (14). A cross-sectional study of advanced CKD and ESKD (33) showed that sleep quality, daytime sleepiness, and restless leg syndrome were associated with profound fatigue. They suggested that sleep disorders might be key to understanding fatigue in CKD and ESKD. In this study, adults with predialysis CKD showed diminished HRQOL, and short or long sleep duration was associated with low HRQOL. Sleep duration has an important association with symptomatic burden in CKD.
Several potential mechanisms may have contributed to the U-shaped association between sleep duration and poor HRQOL. Short sleep duration has adverse effects on neuroendocrine function (5). Neuroendocrine dysfunction can induce fatigue and lethargy in CKD. However, long sleep duration may be associated with uncontrolled comorbidities, such as sarcopenia, malnutrition, and other chronic diseases. In addition, long sleep duration has been linked with sleep fragmentation and depression (34). Short or long sleep duration may be associated with poor HRQOL by different mechanisms (35).
There are several limitations to our study. First, the KNOW-CKD was planned as a prospective observational study, and these results were from an initial cross-sectional study. A cross-sectional study cannot infer the causality. The causation should be confirmed by more rigorous studies. Second, HRQOL assessment is still difficult and complex. There are cultural, geographic, generational, and linguistic variations in the measurement of HRQOL. Universal standard methods to measure the HRQOL are limited. Third, completion of the KDQOL-SF and sleep duration questionnaires was voluntary, and therefore, there could have been potential misclassification. We analyzed the 1910 adults (85%) with predialysis CKD among the 2238 enrolled KNOW-CKD participants. Fourth, self-reported sleep duration might not be objective, and a single measure of sleep duration may not capture the sustained effects on disease outcomes. Actigraphy or polysomnography, which objectively measure sleep duration, was not performed in the study subjects. It is difficult to measure sleep duration, because disagreement between subjective and actigraphic measures of sleep duration has been reported (36), and intraindividual variability of sleep duration has been recognized (37,38). Finally, we cannot rule out the possibility of residual or unmeasured confounders, such as depression, untreated pain, or medication, although we considered various confounding factors in multivariable regression analysis. Despite these limitations, our study has some strengths. The KNOW-CKD is a nationwide CKD study in Korea, and therefore, we were able to collect data from a large and wide sample range. The KDQOL-SF questionnaire might have common clinical applicability, and the measurements are relatively inexpensive. Our study shows an association between self-reported sleep duration and HRQOL in predialysis CKD.
In conclusion, sleep duration had a U-shaped association with low HRQOL, and a 7-hour sleep duration showed the highest HRQOL. Adequate sleep duration has an important association with HRQOL in predialysis CKD. Further studies are necessary to evaluate health outcomes by improving sleep duration in adults with CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the clinical research coordinators of the participating centers for their dedication in patient recruitment and data acquisition.
The Korean Cohort Study for Outcome in Patients with CKD was funded by grants 2011E3300300, 2012E3301100, and 2013E3301600 from Research of the Korea Centers for Disease Control and Prevention.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11351017/-/DCSupplemental.
References
- 1.Jha V, Wang AY, Wang H: The impact of CKD identification in large countries: The burden of illness. Nephrol Dial Transplant 27[Suppl 3]: iii32–iii38, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Grandner MA: Sleep, health, and society. Sleep Med Clin 12: 1–22, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES, Cunningham TJ, Croft JB: Trends in self-reported sleep duration among US adults from 1985 to 2012. Sleep (Basel) 38: 829–832, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ES, Cunningham TJ, Giles WH, Croft JB: Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med 16: 372–378, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turek NF, Ricardo AC, Lash JP: Sleep disturbances as nontraditional risk factors for development and progression of CKD: Review of the evidence. Am J Kidney Dis 60: 823–833, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tokonami N, Mordasini D, Pradervand S, Centeno G, Jouffe C, Maillard M, Bonny O, Gachon F, Gomez RA, Sequeira-Lopez ML, Firsov D: Local renal circadian clocks control fluid-electrolyte homeostasis and BP. J Am Soc Nephrol 25: 1430–1439, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF: Insomnia and risk of cardiovascular disease: A meta-analysis. Eur J Prev Cardiol 21: 57–64, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Liu TZ, Xu C, Rota M, Cai H, Zhang C, Shi MJ, Yuan RX, Weng H, Meng XY, Kwong JS, Sun X: Sleep duration and risk of all-cause mortality: A flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med Rev 32: 28–36, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Maung SC, El Sara A, Chapman C, Cohen D, Cukor D: Sleep disorders and chronic kidney disease. World J Nephrol 5: 224–232, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elder SJ, Pisoni RL, Akizawa T, Fissell R, Andreucci VE, Fukuhara S, Kurokawa K, Rayner HC, Furniss AL, Port FK, Saran R: Sleep quality predicts quality of life and mortality risk in haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 23: 998–1004, 2008 [DOI] [PubMed] [Google Scholar]
- 11.McMullan CJ, Curhan GC, Forman JP: Association of short sleep duration and rapid decline in renal function. Kidney Int 89: 1324–1330, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricardo AC, Knutson K, Chen J, Appel LJ, Bazzano L, Carmona-Powell E, Cohan J, Tamura MK, Steigerwalt S, Thornton JD, Weir M, Turek NF, Rahman M, Van Cauter E, Lash JP; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The association of sleep duration and quality with CKD progression. J Am Soc Nephrol 28: 3708–3715, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyasere O, Brown EA: Determinants of quality of life in advanced kidney disease: Time to screen? Postgrad Med J 90: 340–347, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Chong K, Unruh M: Why does quality of life remain an under-investigated issue in chronic kidney disease and why is it rarely set as an outcome measure in trials in this population? Nephrol Dial Transplant 32[Suppl 2]: ii47–ii52, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Almutary H, Bonner A, Douglas C: Symptom burden in chronic kidney disease: A review of recent literature. J Ren Care 39: 140–150, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Oh KH, Park SK, Park HC, Chin HJ, Chae DW, Choi KH, Han SH, Yoo TH, Lee K, Kim YS, Chung W, Hwang YH, Kim SW, Kim YH, Kang SW, Park BJ, Lee J, Ahn C; Representing KNOW-CKD Study Group : KNOW-CKD (KoreaN cohort study for outcome in patients with chronic kidney disease): Design and methods. BMC Nephrol 15: 80, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin A, Stevens PE: Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 85: 49–61, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB: Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 3: 329–338, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Park HJ, Kim S, Yong JS, Han SS, Yang DH, Meguro M, Han CW, Kohzuki M: Reliability and validity of the Korean version of Kidney Disease Quality of Life instrument (KDQOL-SF). Tohoku J Exp Med 211: 321–329, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Han CW, Lee EJ, Iwaya T, Kataoka H, Kohzuki M: Development of the Korean version of Short-Form 36-Item Health Survey: Health related QOL of healthy elderly people and elderly patients in Korea. Tohoku J Exp Med 203: 189–194, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Consensus Conference Panel; Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E: Joint consensus statement of the American academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: Methodology and discussion. J Clin Sleep Med 11: 931–952, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovás JM: Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv Nutr 6: 648–659, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas SJ, Calhoun D: Sleep, insomnia, and hypertension: Current findings and future directions. J Am Soc Hypertens 11: 122–129, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Lee SWH, Ng KY, Chin WK: The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: A systematic review and meta-analysis. Sleep Med Rev 31: 91–101, 2017 [DOI] [PubMed] [Google Scholar]
- 25.St-Onge MP, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, Bhatt DL; American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council : Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the American heart association. Circulation 134: e367–e386, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindner AV, Novak M, Bohra M, Mucsi I: Insomnia in patients with chronic kidney disease. Semin Nephrol 35: 359–372, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Scherer JS, Combs SA, Brennan F: Sleep disorders, restless legs syndrome, and uremic pruritus: Diagnosis and treatment of common symptoms in dialysis patients. Am J Kidney Dis 69: 117–128, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D: Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A 106: 16523–16528, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M, Bonny O, Firsov D: The circadian clock modulates renal sodium handling. J Am Soc Nephrol 23: 1019–1026, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wuerzner G, Firsov D, Bonny O: Circadian glomerular function: From physiology to molecular and therapeutical aspects. Nephrol Dial Transplant 29: 1475–1480, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Li J, Huang Z, Hou J, Sawyer AM, Wu Z, Cai J, Curhan G, Wu S, Gao X: Sleep and CKD in Chinese adults: A cross-sectional study. Clin J Am Soc Nephrol 12: 885–892, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finkelstein FO, Arsenault KL, Taveras A, Awuah K, Finkelstein SH: Assessing and improving the health-related quality of life of patients with ESRD. Nat Rev Nephrol 8: 718–724, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Jhamb M, Liang K, Yabes J, Steel JL, Dew MA, Shah N, Unruh M: Prevalence and correlates of fatigue in chronic kidney disease and end-stage renal disease: Are sleep disorders a key to understanding fatigue? Am J Nephrol 38: 489–495, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youngstedt SD, Kripke DF: Long sleep and mortality: Rationale for sleep restriction. Sleep Med Rev 8: 159–174, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Knutson KL, Turek FW: The U-shaped association between sleep and health: The 2 peaks do not mean the same thing. Sleep 29: 878–879, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Van Den Berg JF, Van Rooij FJ, Vos H, Tulen JH, Hofman A, Miedema HM, Neven AK, Tiemeier H: Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res 17: 295–302, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS: Intra-individual daily and yearly variability in actigraphically recorded sleep measures: The CARDIA study. Sleep 30: 793–796, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemola S, Ledermann T, Friedman EM: Variability of sleep duration is related to subjective sleep quality and subjective well-being: An actigraphy study. PLoS One 8: e71292, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.