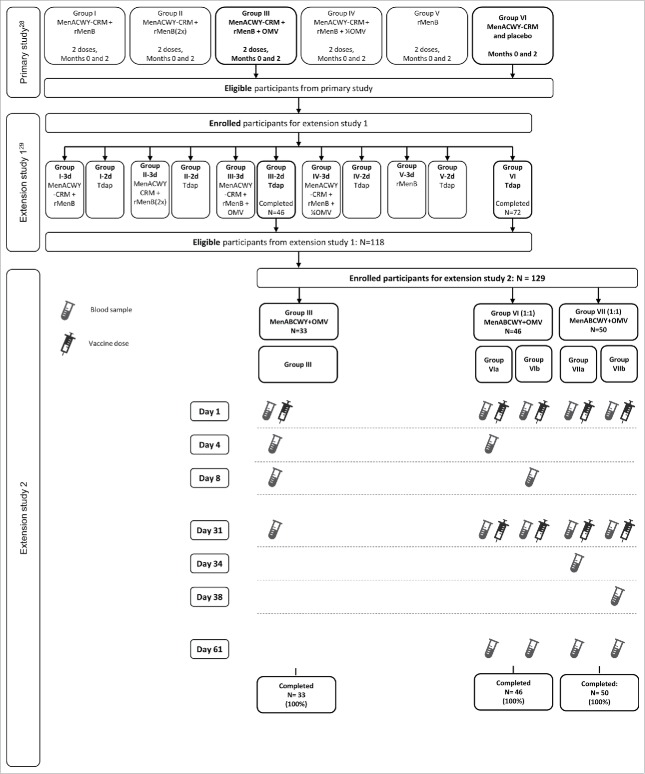
Figure 2.
Flow of participants Footnote: Primary study: participants received 1 or 2 doses of vaccine; Extension study 1: Follow-on participants for 10 months persistence, 3rd dose administration (with control vaccination); Extension study 2: Follow-on participants for 4 years persistence, 3rd dose administration (with control vaccination). Group III; participants received 2 doses of MenABCWY+OMV (presented in this figure, for primary and extension 1 studies, as MenACWY-CRM + rMenB+OMV) in the primary study and 1 dose of MenABCWY+OMV in the extension study presented here (4 years post-primary study). Group VI; participants received 1 dose of MenACWY-CRM in the primary study and 2 doses, 1 month apart, of MenABCWY+OMV in the extension study presented here (4 years post-primary study). Group VII; participants were newly recruited and naïve to vaccination against A, B, C, W and Y serogroups of N. meningitidis. They were vaccinated with 2 doses, 1 month apart, of MenABCWY+OMV in the extension study presented here.