ABSTRACT
Recently, two vaccines against meningococcal serogroup B (MenB) have been developed. They are prepared according to the reverse vaccinology approach and contain 4 (4CMenB) and 2 (MenB-FHbp) cross-reactive surface proteins. In Italy 4CMenB vaccine has been included in the official vaccination schedule only recently and recommended only for infants and toddlers, whereas MenB-FHbp is not licensed. In order to collect information about the present carriage of Neisseria meningitidis serogroup B (MenB) in Italian adolescents and to evaluate the potential protection offered by the presently available MenB vaccines, 2,560 otherwise healthy, high school students aged 14–21 years (907 males, 35.4%, median age 16.2 years) were enrolled in Milan, Italy. A swab to collect posterior pharynx secretions was collected from each subject and meningococcal identification, serogrouping, multilocus sequence typing analysis, sequence alignments and phylogenetic analysis were performed. A total of 135 (5.3%) adolescents were meningococcal carriers. Strains belonging to serogroup B were the most common (n = 58; 2.3%), followed by MenY (n = 32; 1.2%), MenC (n = 7; 0.3%), MenW (n = 6; 0.3%) and MenX (n = 5; 0.2%). The remaining bacteria were not capsulated. The identified MenB strains belonged to eleven clonal complexes (CCs): ST-162 CC (n = 12; 20.7%), ST-865 CC (n = 12; 20.7%), ST-41/44/Lin.3 CC (n = 11; 19.0%), ST-35 CC (n = 6; 10.3%), ST-32/ET-5 CC (n = 4; 6.9%), ST-269 CC (n = 3; 5.2%), ST-213 CC (n = 2; 3.4%), ST-198 CC (n = 1; 1.7%), ST-461 CC (n = 1; 1.7%), ST-549 CC (n = 1; 1.7%), and ST-750 CC (n = 1; 1.7%). This study showed that MenB was the most commonly carried meningococcal serogroup found in adolescents living in Milan, Italy. The MenB vaccines presently licensed could have theoretically induced the production of antibodies effective against the greatest part of the identified MenB strains (100% in the case of 4CMenB and 95% in case of MenB-FHbp) Monitoring carriage remains essential to evaluate MenB circulation, but further studies are necessary to evaluate the effect on carriage and the final efficacy of both new MenB vaccines.
KEYWORDS: Epidemiology, MenB, meningococcal prevention, meningococcal vaccines, Neisseria meningitidis
Introduction
Nasopharyngeal carriage of Neisseria meningitidis is relatively common. It occurs in 3–25% of the general population,1 with the highest rates in adolescents and young adults mainly because of the highest contact rates among people of these age groups.2 Usually, carriage is not associated with disease development. However, in very few cases, shortly after colonization, N. meningitidis can pass through the respiratory mucosa, enter the blood stream and cause invasive meningococcal disease (IMD), mainly sepsis and meningitis.3 The risk of IMD is higher in subjects without circulating protective bactericidal antibodies and in those with complement deficiencies.4 In addition, bacterial characteristics may play a role in this regard. With very few exceptions,5,6 all the cases of IMD are due to encapsulated meningococcal strains. Moreover, only 6 of the known 12 capsule variants are associated with IMD. Finally, within the potentially invasive variants, the development of IMD is more common with strains of specific genetic types.7
Meningococcal carriage represents a critical condition for IMD onset since it represents the first step for disease transmission. Moreover, high levels of carriage can favour genetic exchange among meningococcal strains,7 leading to the development of bacteria with invasive potential.8 Thus, meningococcal carriage epidemiology is important in understanding relationships between carriage and disease.9 Moreover, it has been shown that the meningococcal strains carried by adolescents have similar genetic characteristics to those that cause disease in infants.10 Consequently, knowledge of carriage characteristics can, at least in part, inform on the meningococcal strains that can cause IMD in a given period and in a geographic area. Furthermore, the level of protection of meningococcal vaccines might be indirectly deduced by the evaluation of the carriage.
Recently, two vaccines against meningococcal serogroup B (MenB) have been developed. They are prepared according to the reverse vaccinology approach and contain 4 (4CMenB) and 2 (MenB-FHbp) relatively well-conserved and potentially cross-reactive surface proteins.11,12 Both were found able to evoke antibodies effective in killing Men strains in vitro.11,12 Moreover, particularly for 4CMenB, evidence of the efficacy in humans has been collected.13,14 In Italy 4CMenB vaccine has been included in the official vaccination schedule only recently and recommended only for infants and toddlers, although a possible inclusion for adolescent in the future was suggested.20 Furthermore, MenB-FHbp is not licensed. The main aim of this study was to collect information about the present carriage of MenB in Italian adolescents and to evaluate the potential protection offered by the presently available MenB vaccines. Because a similar study was performed in 2011,15 a comparison with the previously collected data was also performed.
Results
Study population
In this study, 2,560 otherwise healthy students aged 14–21 years (907 males, 35.4%, median age 16.2 years) were enrolled. A total of 135 (5.3%) were meningococcal carriers. Among those who carried bacteria, strains belonging to serogroup B were the most common (n = 58; 2.3%), followed by MenY (n = 32; 1.2%), MenC (n = 7; 0.3%), MenW (n = 6; 0.3%) and MenX (n = 5; 0.2%). The remaining bacteria were not capsulated (n = 27; 1.0%). No differences in Men carriage prevalence according to type of school, age and gender was evidenced. Unfortunately, only a marginal part of the enrolled students returned the questionnaire. Consequently, no relationship between characteristics of the students and Men carriage could be studied.
Clonal complex (CC)
The identified 58 MenB strains belonged to eleven CCs: ST-162 CC (n = 12; 20.7%), ST-865 CC (n = 12; 20.7%), ST-41/44/Lin.3 CC (n = 11; 19.0%), ST-35 CC (n = 6; 10.3%), ST-32/ET-5 CC (n = 4; 6.9%), ST-269 CC (n = 3; 5.2%), ST-213 CC (n = 2; 3.4%), ST-198 CC (n = 1; 1.7%), ST-461 CC (n = 1; 1.7%), ST-549 CC (n = 1; 1.7%), and ST-750 CC (n = 1; 1.7%). Four strains (6.9%) belonged to sequence types that have not yet been assigned to any CC by the Meningococcal Multilocus Sequence Typing System (http://pubmlst.org/neisseria).
Gene analysis
fHbp
Figure 1 shows the distribution of the fHbp variants by CC. All but three of the detected MenB strains harboured fHbp alleles, representing a total of 31 sub-variants: 18 in variant 1 (v.1 n = 25; 45.4%) and 13 in variants 2 and 3 (8 in v.2, n = 25, 45.4%; 5 in v. 3, n = 5, 9.1%). Variants 1 and 2 were mainly present in the ST-162, ST41/44/Lin3, and ST-865 CCs; variant 3 was mainly found in ST-269 CC.
Figure 1.
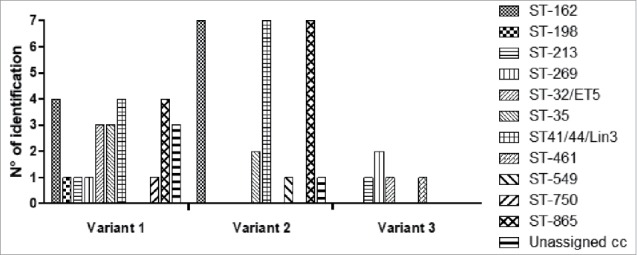
Distribution of the fHpb variant by clonal complex.
Among the 18 sub-variants in variant 1, the gene for the fHbp sub-variant included in the 4CMenB vaccine (fHbp 1.1) was not identified in any strain. Regarding the genes for the sub-variants included in the 2CMenB vaccine, fHbp 1.55 was not detected in any studied strain, whereas fHbp 3.45 was detected in one strain.
nhba
The gene for the nhba protein was found in all but 6 strains (10.3%), and 21 sub-variants were identified. The gene for the nhba protein included in the 4CMenB vaccine (sub-variant 2) was not found in any of the strains; among the sub-variants, the most common were 908 (n = 10; 19.2%) in ST-865 CC (9 cases) and in ST- 549 (one case); 879 (n = 10; 19.2%) in ST-162 CC (9 cases) and in ST-865 CC (one case); and 21 (n = 6; 11.5%) in ST-35 CC (4 cases) and in ST-269 CC (2 cases). Among the strains without the nhba gene, 5 had the gene for fHbp protein variant 1 (sub-variants 1.45, 1.464, 1.6, and 1.66), and 1 had the gene for fHbp protein variant 2 (sub-variant 2.19).
PorA
PorA proteins were identified in 53 of the 58 (91.4%) studied MenB strains. In the remaining 5 strains PorA was deleted There were 22 porA sub-types. The protein included in the 4CMenB vaccine (P1 7–2,4) was identified in 4 cases belonging to ST-162CC (3 strains) and ST-41/44 Lin3CC (1 strain). Among the other sub-types, the most common were P1 21,16–36 (n = 10; 18.8%) in ST-865 CC (8 strains) and ST-549 CC (2 strains); P1 22,14 (n = 10; 18.8%) in ST-162 CC (6 strains), ST-213 CC (2 strains), ST-750 CC (1 strain), and ST-865 (1 strain); P1 22–1,14 (n = 5; 9.4%) in ST-35 CC; P1 18,25 (n = 4; 7.5%) in ST-41/44 Lin3 CC (4 strains); P1 7–1,1 (n = 3; 5.6%) in ST-32 (2 strains) and ST-41/44 Lin3 (1 strain); and P1 18–1,30 (n = 2; 3.7%) in ST-32 CC. All of the other PorA subtypes were represented in a single CC.
Global characteristics of identified MenB strains
All of the studied MenB strain had at least one of the genes encoding the proteins included in 4CMenB vaccine. Three genes were identified in 19 meningococci, two in 34 (1 fHbp with nhab, 3 fHbp with PorA and 30 nhba with PorA). Finally, n 5 strains only one gene (2 fHbp, 2 nhba and 1 PorA) were detected. Regarding proteins included in MenB-FHbp vaccine, 25 strains had the fHbp1, 25 the fHbp2 and 5 tha fHbp3 genes.
NadA
The gene for the NadA protein was not detected in any of the 58 studied strains.
Discussion
In this study, MenB was the most commonly carried meningococcal serogroup found in adolescents living in Milan, Italy, followed by MenY. This finding agrees with recently reported studies in Europe in which MenB is presently the most frequently identified meningococcus in the pharynx of both healthy individuals and patients with IMD.16-18 This was expected because the introduction of the MenC conjugate vaccine has significantly reduced MenC carriage and circulation, evidencing the role of MenB in carriage and disease.16-18 In Northern Italy, MenC vaccine coverage in children younger than 24 months of age is about 90%19 and this could explain the low detection of MenC in the studied population. On the contrary, in Italy 4CMenB vaccine has been included in the official vaccination schedule only recently and recommended only for infants and toddlers, although a possible inclusion for adolescent in the future was suggested.20 Moreover, MenB-FHbp is not licensed. This means that no effect of MenB vaccination may have occurred. On the other hand, in Lombardy, the Region of Italy where this study was carried out, incidence of MenB invasive disease has remained substantially unmodified in the last 10 years (i.e., around 4 cases out of 100,000).,21
The genetic characteristics of MenB strains identified in this study are quite similar to those of the strains identified in a previous study performed several years earlier in a similar population living in the same geographic area.15 This seems to confirm that, despite the high incidence of genetic exchange, meningococcal strains are relatively stable.7 In both cases, many strains were included in some of the CC frequently associated with bacterial hypervirulence and the development of IMD, such as ST-41/44 and ST-32.22 This suggests a potential virulence of the meningococcal strains carried by the study population.
Moreover, similar to the previous study,15 the MenB vaccines presently licensed could In theoretically induced the production of antibodies effective against the greatest part of the identified MenB strains, although with slight differences between them. The 2CMenB vaccine contains two sub-variants of the bacterial fHbp protein (fHbp1 and fHbp3) and one of the genes encoding these proteins was found in 55/58 studied strains. This seems to indicate that, the MenB.fHbp vaccine could have evoked antibodies able to eliminate the carried strains in about 95% of the cases. The 4CMenB vaccine contains a variant of the fHbp protein (sub-variant 1.1), a NadA-3 component, a variant of the nhba protein (sub-variant 2), and the Por A protein (subtype P1 7–2,4). In the studied bacteria, the gene for the NadA was never found, as frequently observed in carried bacteria.23 This seems to indicate that this component of the 4CMenB vaccine could not have a role in eliminating carried pathogens. However, all the other genes were identified in most of the meningococci. Two or 3 of the genes encoding the proteins included in the vaccine were identified in more than 80% of the cases.
Although carried isolates not always related to the invasive strains occurring during the study period, elimination of carriage seems essential to maximize the efficacy of a meningococcal vaccine. A relevant part of the positive effects obtained with the use of MenC conjugate vaccine were due to population immunity induced by the reduction of carriage and pathogen transmission.24 However, the potential positive effect of both MenB vaccines suggested by this study should be considered with caution. Only the presence of genes encoding proteins included in the vaccines was studied. Gene expression was not evaluated, and it has been demonstrated that MenB vaccines are theoretically effective when the invasive Men strain is able to produce one or more of the vaccine components at a concentration higher than minimum required for the bactericidal protective activities of antibodies.11,12 Conversely, data collected in this study seem to disagree, at least in part, with what has been reported by Read et al.17 These authors reported that although there was no significant difference in carriage between controls and 4CMenB vaccinated subjects at 1 month after vaccination, at 3 months after dose two, 4CMenB vaccination resulted in 18.2% lower carriage of any meningococcal strain including the capsular groups BCWY, for which carriage reduction was 26.6%.17 This finding was not surprising, as the proteins included in both the 2CMenB and 4CMenB vaccines are not exclusive of MenB but are present in meningococcal isolates regardless of the serogroup. However, data collected by Read et al. regard a relatively small group of subjects and do not allow to draw firm conclusions.17 On the other hand, more recent studies have reported that neither vaccine used to control a college outbreak of MenB IMD was able to rapidly reduce meningococcal carriage or prevent MenB carriage acquisition.25,26 Therefore, monitoring carriage remains essential to evaluate MenB circulation, but further studies are necessary to evaluate the effect on carriage and the final efficacy of both new MenB vaccines.
Materials and methods
This study was conducted in Milan, Italy, between January and March 2016, in the middle of the school year Healthy participants were recruited among students attending four high schools. Enrolment was voluntary. However, in the week preceding the swabbing, the students received a brochure providing information regarding meningococcal clinical relevance and the purpose of the study. Moreover, during lesson time, science teachers reinforced the message. Contemporaneously to the execution of the throat swab, date regarding age and sex of the participants were collected. in addition, each student was asked to fill in a questionnaire at home to provide information on personal and family characteristics to be returned to the teacher of science within a week. This study was conducted in compliance with the ethical principles of the Helsinki Declaration within the guidelines of Good Clinical Practice. It was approved by the Ethical Committee of the Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy. Written informed consent was obtained from all participants and from both parents of those <18 years old.
The posterior oropharynx secretions were collected in the medical rooms of the participating schools by a group of experienced nurses supervised by a paediatrician (SE). ESwab kits (code 482CE, Copan Italia) were used for swabbing. The samples were immediately transported to a central laboratory and processed within 3 hours. Bacteria identification, serogrouping (A, B, C, X, Y, Z, W, E), multilocus sequence typing (MLST) analysis, sequence alignments and phylogenetic analysis were performed. The nomenclature for the fHbp and PorA sub-variants used in this study follows that of the public fHbp and PorA database (http://neisseria.org) in which new allele sub-variants are assigned a sequentially allocated numerical identifier and a pre-existing or new (sequentially allocated) numerical protein identifier. The PorA variable region types were described on the basis of the nomenclature provided in the N. meningitidis PorA VR database (http://pubmlst.org/neisseria/ PorA/). The nhba and nadA genes were sequenced using the protocols and nomenclature provided in the N. meningitidis pubMLST database (http://pubmlst.org/neisseria).
Funding Statement
This review was supported by an unrestricted grant from the World Association for Infectious Diseases and Immunological Disorders (WAidid) and a grant from the Italian Ministry of Health (Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Ricerca Corrente 2017 850/02).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–61. doi: 10.1016/S1473-3099(10)70251-6. PMID:21075057. [DOI] [PubMed] [Google Scholar]
- 2.MacLennan J, Kafatos G, Neal K, Andrews N, Cameron JC, Roberts R, Evans MR, Cann K, Baxter DN, Maiden MC, et al.. Social behavior and meningococcal carriage in British teenagers. Emerg Infect Dis. 2006;12:950–7. doi: 10.3201/eid1206.051297. PMID:16707051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coureuil M, Join-Lambert O, Lécuyer H, Bourdoulous S, Marullo S, Nassif X. Mechanism of meningeal invasion by Neisseria meningitidis. Virulence. 2012;3:164–72. doi: 10.4161/viru.18639. PMID:22366962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010;23:740–80. doi: 10.1128/CMR.00048-09. PMID:20930072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoang LM, Thomas E, Tyler S, Pollard AJ, Stephens G, Gustafson L, McNabb A, Pocock I, Tsang R, Tan R. Rapid and fatal meningococcal disease due to a strain of Neisseria meningitidis containing the capsule null locus. Clin Infect Dis. 2005;40:e38–42. doi: 10.1086/427875. PMID:15714405. [DOI] [PubMed] [Google Scholar]
- 6.Findlow H, Vogel U, Mueller JE, Curry A, Njanpop-Lafourcade BM, Claus H, Gray SJ, Yaro S, Traoré Y, Sangaré L, et al.. Three cases of invasive meningococcal disease caused by a capsule null locus strain circulating among healthy carriers in Burkina Faso. J Infect Dis. 2007;195:1071–7. doi: 10.1086/512084. PMID:17330799. [DOI] [PubMed] [Google Scholar]
- 7.Caugant DA, Maiden MC. Meningococcal carriage and disease–population biology and evolution. Vaccine. 2009;27Suppl 2:B64–70. doi: 10.1016/j.vaccine.2009.04.061. PMID:19464092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph B, Schwarz RF, Linke B, Blom J, Becker A, Claus H, Goesmann A, Frosch M, Müller T, Vogel U, et al.. Virulence evolution of the human pathogen Neisseria meningitidis by recombination in the core and accessory genome. PLoS One. 2011;6:e18441. doi: 10.1371/journal.pone.0018441. PMID:21541312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianchecchi E, Piccini G, Torelli A, Rappuoli R, Montomoli E. An unwanted guest: Neisseria meningitidis – carriage, risk for invasive disease and the impact of vaccination with insight on Italy incidence. Expert Rev Anti Infect Ther. 2017;15:689–701. doi: 10.1080/14787210.2017.1333422. PMID:28524748. [DOI] [PubMed] [Google Scholar]
- 10.Anderson AS, Hao L, Jiang Q, Harris SL, Jones TR, Perez JL, York L, Eiden J, Jansen KU. Potential impact of the bivalent rLP2806 vaccine on Neisseria meningitidis carriage and invasive serogroup B disease. Hum Vaccin Immunother. 2013;9:471–9. doi: 10.4161/hv.23222. PMID:23249817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Medicines Agency Bexero. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002333/human_med_001614.jsp&mid=WC0b01ac058001d124. Accessed on: October9, 2017. [Google Scholar]
- 12.Patton ME, Stephens D, Moore K, MacNeil JR. Updated recommendations for use of MenB-FHbp serogroup B meningococcal vaccine – Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:509–13. doi: 10.15585/mmwr.mm6619a6. PMID:28520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson PS, Turner DP. Clinical experience with the meningococcal B vaccine, Bexsero(®): Prospects for reducing the burden of meningococcal serogroup B disease. Vaccine. 2016;34:875–80. doi: 10.1016/j.vaccine.2015.11.057. PMID:26686570. [DOI] [PubMed] [Google Scholar]
- 14.Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, White JM, Borrow R, Ramsay ME, Ladhani SN. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388:2775–82. doi: 10.1016/S0140-6736(16)31921-3. PMID:28100432. [DOI] [PubMed] [Google Scholar]
- 15.Esposito S, Zampiero A, Terranova L, Montinaro V, Scala A, Ansuini V, Principi N. Genetic characteristics of Neisseria meningitidis serogroup B strains carried by adolescents living in Milan, Italy: implications for vaccine efficacy. Hum Vaccin Immunother. 2013;9:2296–303. doi: 10.4161/hv.25800. PMID:23880917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeppesen CA, Snape MD, Robinson H, Gossger N, John TM, Voysey M, Ladhani S, Okike IO, Oeser C, Kent A, et al.. Meningococcal carriage in adolescents in the United Kingdom to inform timing of an adolescent vaccination strategy. J Infect. 2015;71:43–52. doi: 10.1016/j.jinf.2015.02.006. PMID:25709085. [DOI] [PubMed] [Google Scholar]
- 17.Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, Heath PT, Lewis DJ, Pollard AJ, Turner DP, et al.. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384:2123–31. doi: 10.1016/S0140-6736(14)60842-4. PMID:25145775. [DOI] [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control Invasive meningococcal disease – Annual Epidemiological Report 2016 [2014 data]. Available at: https://ecdc.europa.eu/en/publications-data/invasive-meningococcal-disease-annual-epidemiological-report-2016-2014-data Accessed on: October9, 2017.
- 19.Ministero della salute Istituto Superiore di sanita'. Copetura vaccinale in Italia. Meningococco C. Available at: http://www.epicentro.iss.it/temi/vaccinazioni/dati_Ita.asp Accessed on: December11, 2017.
- 20.Ministero della Salute Piano Nazionale Prevenzione Vaccinale PNPV 2017–2019. Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf Accessed on: December11, 2017.
- 21.Istituto Superiore di Sanità Dati di sorveglianza delle malattie batteriche invasive aggiornati al 16 novembre 2016. Available at: http://www.iss.it/binary/mabi/cont/Report_MBI_20161116_v11.pdf. Accessed on: December14, 2017.
- 22.Racloz VN, Luiz SJ. The elusive meningococcal meningitis serogroup: a systematic review of serogroup B epidemiology. BMC Infect Dis. 2010;10:175. doi: 10.1186/1471-2334-10-175. PMID:20565757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comanducci M, Bambini S, Caugant DA, Mora M, Brunelli B, Capecchi B, Ciucchi L, Rappuoli R, Pizza M. NadA diversity and carriage in Neisseria meningitidis. Infect Immun. 2004;72:4217–23. doi: 10.1128/IAI.72.7.4217-4223.2004. PMID:15213166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maiden MC, Ibarz-Pavón AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, et al.. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. doi: 10.1086/527401. PMID:18271745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soeters HM, Whaley M, Alexander-Scott N, Kanadanian KV, MacNeil JR, Martin SW, McNamara LA, Sicard K, Vanner C, Vuong J, et al.. Meningococcal carriage evaluation in response to a serogroup B meningococcal disease outbreak and mass vaccination campaign at a college-Rhode Island, 2015–2016. Clin Infect Dis. 2017;64:1115–22. doi: 10.1093/cid/cix091. PMID:28158417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNamara LA, Thomas JD, MacNeil J, Chang HY, Day M, Fisher E, Martin S, Poissant T, Schmink SE, Steward-Clark E, et al.. Meningococcal carriage following a University serogroup B meningococcal disease outbreak and vaccination campaign with MenB-4C and MenB-FHbp – Oregon, 2015–6. J Infect Dis. 2017;216:1130–40. Epub Aug 26. doi: 10.1093/infdis/jix446. [DOI] [PMC free article] [PubMed] [Google Scholar]


