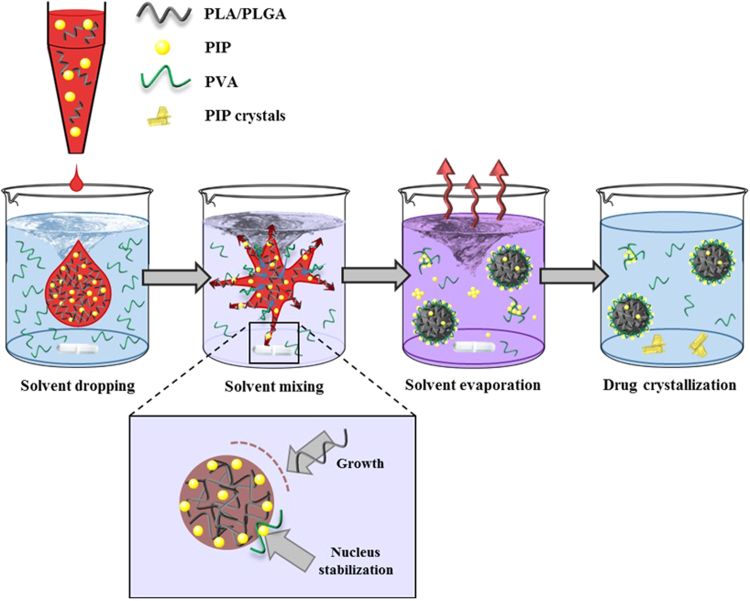
Figure 1.
Schematic representation of nanoprecipitation process to obtain PLA/PLGA PIP-loaded NPs. The organic phase (red) containing the polymer (PLA/PLGA) and PIP is poured dropwise into an aqueous solution (blue) containing PVA as surfactant, under vigorous magnetic stirring. Once the drop enters in contact with the aqueous phase, the two solvents are mixed together (purple color) causing the “explosion” of the organic solvent drop. The local fluctuations in the supersaturated system containing the polymer and PIP led to the spontaneous formation of nucleation sites. If the nuclei further encounter more polymer chains, newly formed NPs tend to grow (lower panel). When the polymer concentration decreases, the probability of NP growth decreases too. When the nuclei encounter PVA molecules, PIP and PVA associate at the nucleus surface stopping its growth and leading to a stabilization effect, eventually leading to NP formation. Finally, NP suspension is maintained under magnetic stirring to allow organic solvents evaporation. In turn, solvent evaporation decreases PIP solubility in the suspension medium. PIP molecules which are not stabilized neither on the NPs surface by association to PVA nor by free PVA, crystalize at the bottom of the vial.