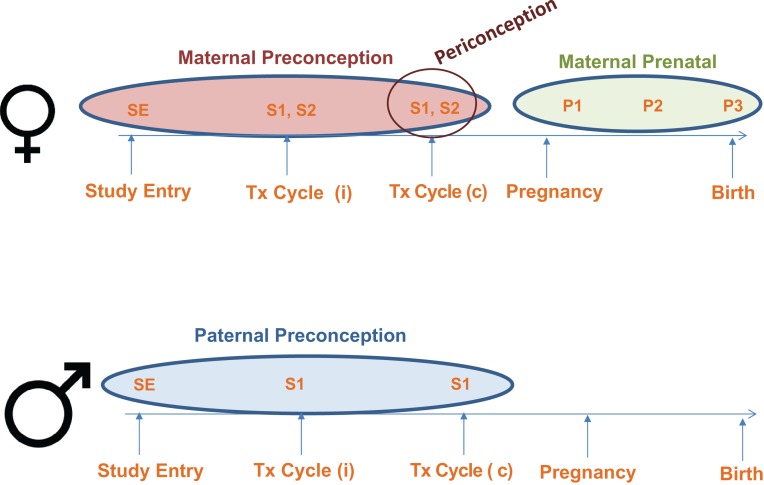
Figure 1.
Maternal and Paternal Assessment in the Environment and Reproductive Health (EARTH) Study. Female participants: Study entry (SE) assessment includes: baseline urine and blood samples, and completion of the baseline and full questionnaires (includes the Food Frequency Questionnaire). Treatment (Tx) cycle (i) denotes any number of followed cycles including those treated with IVF-based technologies or non-IVF based procedures. Assessment at two points in time during each Tx cycle: S1—includes the first spot urine sample and blood sample collected during the follicular phase of the cycle (Days 3–9) and the completion of the Product Use Questionnaire at the same point in time. S2—includes the second spot urine sample collected at the time of scheduled treatment procedure (oocyte retrieval, embryo transfer or IUI) and a follicular fluid sample collected during oocyte retrievals. All SE, S1 and S2 samples represent exposure in the maternal preconception period. Tx (c) denotes the index cycle of conception. Clinical information about the mode of conception (IVF-based, non-IVF based or non-medically assisted) is abstracted from electronic medical records by trained study staff. S1 and S2 samples collected in the index conception represent exposure in the maternal periconception period. P1/P2/P3—includes a single urine sample and blood sample and Produce Use Questionnaires collected in the first, second and third trimester of pregnancy, respectively. P1, P2 and P3 samples collected following the index conception represent the maternal prenatal exposure period. Male participants: SE assessment includes: baseline urine and blood samples, and completion of the Baseline and Full Questionnaires (includes the Food Frequency Questionnaire). Men also provide a semen sample and an abstinence time questionnaire at baseline if their study entry visit coincides with a routine semen sample collection. Assessment at Tx cycle: S1 includes a spot urine sample, blood sample and semen sample along with the abstinence time questionnaire on the day their female partner undergoes their scheduled fertility treatment procedure. SE and S1 samples collected up to the index conception represent the paternal preconception exposure period.