Fig. 1.
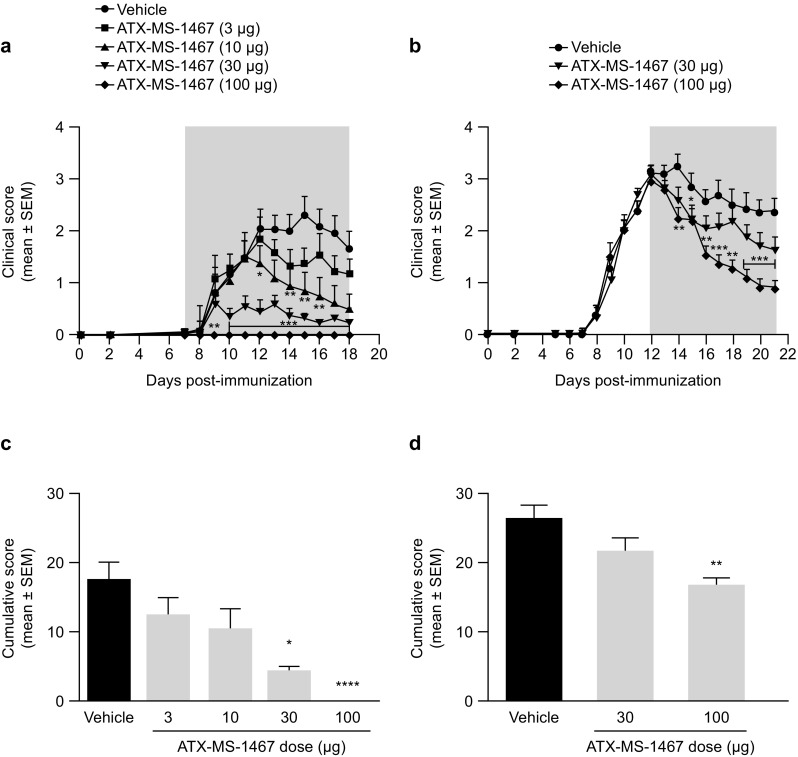
Effect of dose response of ATX-MS-1467 from disease onset or peak of clinical disability in a mouse model of EAE. EAE was induced at 0 dpi with spinal cord homogenate in (DR2 × Ob1)F1 mice, and treatment was initiated with ATX-MS-1467 at 7 dpi (a, c) or at 12 dpi (b, d) at doses ranging 3–100 µg, as indicated, subcutaneously three times per week. The grey area in graphs a, b indicates the treatment period. For the experiment shown in a, c, the group sizes were n = 10–14 mice, and for the experiment shown B and D, the data represent a pool from two independent experiments with a total of n = 21–22 mice. *, **, *** and **** indicate p < 0.05, < 0.01, < 0.001 and < 0.0001, respectively, by Kruskal–Wallis test followed by Dunn’s test versus vehicle (PBS) group. dpi days post-injection, EAE experimental autoimmune encephalomyelitis, PBS phosphate-buffered saline, SEM standard error of the mean
