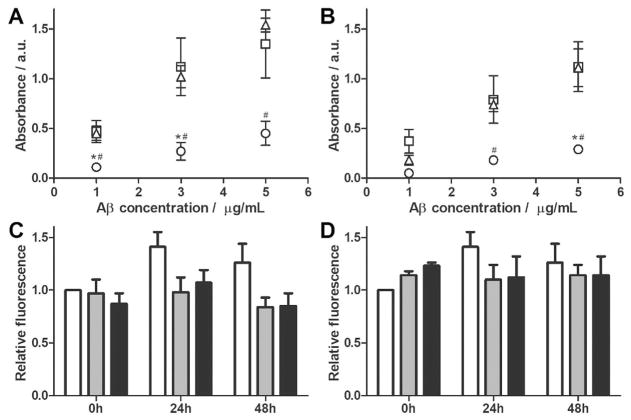
Figure 4.
cG8 and mTTR bind to multiple Aβ species but do not disaggregate Aβ fibrils. (A–B) NeutrAvidin plates were coated with 5 μgmL−1 of either cG8b (A) or mTTRb (B) and the relative binding of Aβ monomer (fresh, ○), oligomer (24 h, RT, □) or fibril (24 h, 37°C, △) was measured. Data was blank (PBS, 0 mgmL−1 Aβ) subtracted. Error bars indicate SEM, n=3. *p<0.05 vs. oligomer. #p<0.05 vs. fibril. (C–D) Preformed Aβ fibrils were combined with cG8 (C) or mTTR (D). Final samples contained 28 μM Aβ alone (white bars), or at Aβ/inhibitor molar ratios of 2:1 (grey bars), or 1:1 (black bars). Disaggregation was monitored via ThT fluorescence and data was normalized to the fluorescence of the control at 0 h. Error bars indicate SEM, n=3 for samples, n=6 for control.