Short abstract
Objective
Medullary thyroid carcinoma (MTC) is classified as either sporadic or inherited. This study was performed to analyze the risk factors for cervical lymph node metastases and predict the indication for prophylactic lateral neck dissection in patients with sporadic MTC.
Methods
Sixty-five patients with sporadic MTC were retrospectively reviewed. Univariate analysis with the chi-square test and multiple logistic regression analysis were applied to identify the clinicopathological features (sex, age, tumor size, number of tumor foci, capsule or vascular invasion, and others) associated with cervical lymph node metastases.
Results
The metastasis rates in the central and lateral compartments were 46.2% (30/65) and 40.0% (26/65), respectively. The incidence of cervical lymph node metastases was significantly higher in patients with a tumor size of >1 cm, tumor multifocality, and thyroid capsule invasion. Only thyroid capsule invasion was an independent predictive factor for central compartment metastases and lateral neck metastases. The possibility of central compartment metastases was significantly higher when the preoperative serum carcinoembryonic antigen concentration was >30 ng/mL (60.0% vs. 34.3%).
Conclusions
MTC is associated with a high incidence of cervical lymph node metastases. Prophylactic lateral node dissection is necessary in patients with thyroid capsule invasion or a high serum carcinoembryonic antigen concentration.
Keywords: Thyroid neoplasm, carcinoma, medullary, lymph node metastases, node dissection, carcinoembryonic antigen
Introduction
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor of the thyroid gland that is derived from parafollicular C cells and was first described in 1959.1 It accounts for 5% to 10% of all thyroid cancers and may be classified as either hereditary MTC (25%) or sporadic MTC (SMTC) (75%).1–4 Parafollicular C cells can produce calcitonin and carcinoembryonic antigen (CEA), which are relatively sensitive and specific markers for both the preoperative diagnosis and follow-up of MTC.1,5,6 Due to the lack of treatment options other than surgery, the standard treatment for MTC is total thyroidectomy with prophylactic central lymph node dissection in clinically node-negative patients and therapeutic neck dissection in clinically suspicious lymph node-positive patients.7–10 However, controversy remains surrounding the indication for prophylactic lateral lymph node dissection. This study was performed to analyze the risk factors for cervical lymph node metastases and predict the indication for prophylactic lateral neck dissection in patients with SMTC.
Materials and methods
Study population
We retrospectively studied the medical records of patients who were diagnosed with SMTC from 2007 to 2017 in the First Affiliated Hospital of Zhejiang University, China. All patients underwent preoperative ultrasound (including evaluation of the thyroid bed, central compartment, and bilateral lateral neck), preoperative measurement of CEA levels, and/or computed tomography neck imaging. Patients with a clinical suspicion of SMTC underwent fine-needle aspiration of thyroid nodules. Only patients with postoperative histopathological features consistent with the diagnosis of SMTC were included in the study. We excluded patients with SMTC who had distant metastases. The following characteristics of each patient were recorded: age at diagnosis of SMTC, sex, tumor size, number of tumor foci (unifocality/multifocality), capsule or vascular invasion, lymph node metastases, and extrathyroidal extension of the tumor. According to the National Comprehensive Cancer Network (NCCN) guidelines in 2017, tumor size was categorized as ≥1 cm or <1 cm. Because MTC is often associated with multiple endocrine neoplasia with diagnosis at a younger age, the patients were stratified into two relevant groups: those aged <45 and ≥45 years. The stage of MTC was defined based on the NCCN tumor-node-metastasis (TNM) classification system. All participants were well informed about the aim of the study, and all agreed to participate in the study. The study was approved by the ethics committee of the First Affiliated Hospital of Zhejiang University, Hangzhou, China.
Histopathological examination
All patients’ thyroid glands were available for histopathological examination. After fixation in formalin, the whole thyroid gland was embedded in paraffin. Soft tissue and lymph nodes were processed separately. Conventional staining (hematoxylin and eosin) and calcitonin immunohistochemistry were performed on every surgical specimen using the standard avidin-biotin complex peroxidase approach and a commercial monoclonal antibody (ZSGB-BIO, Beijing, China). The diagnosis of SMTC was confirmed by positive immunostaining for calcitonin and CEA in all patients. Two experienced pathologists assessed the characteristics of the tumor, including the size, unifocality or multifocality, unilaterality or bilaterality, extrathyroidal extension of the tumor, demonstration of lymphatic or vascular invasion, or a combination thereof. The primary tumor diameter was ascertained by direct measurements of the surgical thyroid specimens. When multiple MTCs were present, only the largest tumor was considered. Multifocality was defined as more than one tumor lesion, regardless of unilaterality or bilaterality.
Surgical treatment
The surgical procedure was performed with curative intent in all patients. All patients underwent total thyroidectomy with lymph node dissection of the central neck compartment (level VI). Patients with tumors of ≥1 cm in diameter as well as both clinical and radiological validation of lymph node involvement underwent therapeutic ipsilateral or bilateral modified neck dissection (levels II–V). In contrast, prophylactic central neck dissection was performed for patients with no clinical or radiological evidence of identifiable cervical node metastases.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics, version 22.0 (IBM Corp., Armonk, NY, USA). Data are presented as mean ± standard deviation or median (range), where appropriate. Associations between lymph node involvement and tumor characteristics were evaluated using the chi-square test. Logistic regression analysis was used to identify whether the factors that were significant in the univariate analysis were still statistically significant in the multivariate analysis. In all cases, a p value of <0.05 was considered significant.
Results
In total, 65 patients (27 male, 38 female) with SMTC were included in this study. Their mean age at diagnosis was 49.7 ± 12.6 years (range, 22–76 years). The detailed clinicopathological characteristics of the 65 patients are listed in Table 1. The mean tumor size was 2.0 ± 1.3 cm. Among all 65 patients, 17 (26.2%) were had multifocal tumors and 38 had unifocal tumors. Lymph node metastases were found in 34 of 65 patients (54.3%), including 30 (46.2%) patients with central cervical lymph node involvement and 26 (40.0%) with lateral lymph node metastases. In the absence of positive central cervical lymph nodes, 3 of 65 patients had positive nodes (skip metastases) in the ipsilateral lateral neck compartment. The lateral neck lymph nodes were systematically dissected in 35 patients (53.8%). Three patients with bilateral thyroid lesions underwent bilateral radical lymph node dissection. Upper mediastinal dissection was performed by sternotomy in only one patient with macroscopic central node involvement. According to the NCCN guidelines, pathological TNM staging showed that 19 patients had stage I disease at presentation, 8 had stage II, 7 had stage III, and 31 had stage IV.
Table 1.
Clinicopathological and pathological characteristics of 65 patients with sporadic medullary thyroid carcinoma.
| Characteristic | |
|---|---|
| Sex, female/male | 27/38 |
| Age at diagnosis, years | 49.7 ± 12.6 |
| Tumor size, cm | 2.0 ± 1.3 |
| Multifocal tumors | 17 (26.2) |
| Thyroid capsule invasion | 21 (32.3) |
| Vascular invasion | 22 (33.8) |
| Lymph node metastases | 34 (52.3) |
| Infiltration of surrounding soft tissues | 16 (24.6) |
| CEA level, ng/mL | |
| <30 | 35 (53.8) |
| ≥30 | 30 (46.2) |
| Pathological TNM stage | |
| I | 19 (29.2) |
| II | 8 (12.3) |
| III | 7 (10.8) |
| IV | 31 (47.7) |
Data are presented as n, n (%), or mean ± standard deviation. CEA, carcinoembryonic antigen; TNM: tumor-node-metastasis
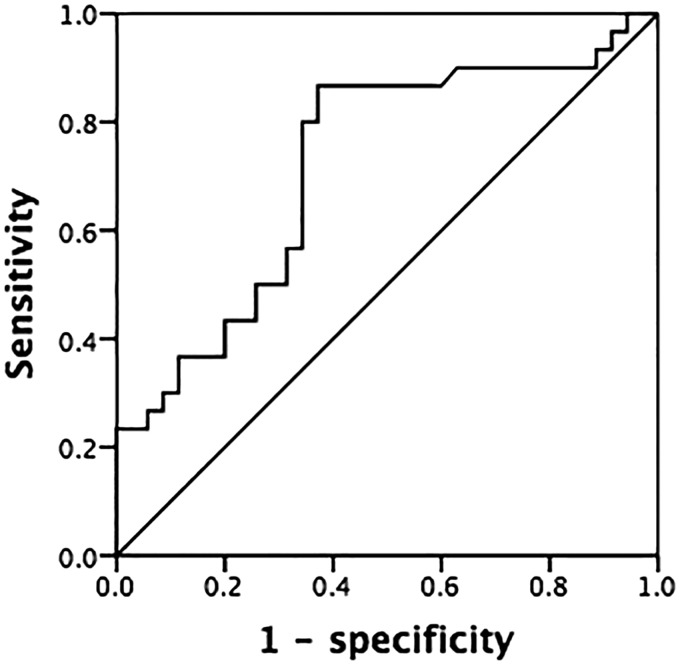
All variables that were of statistical significance in the univariate logistic regression (p < 0.05) were incorporated into a logistic regression analysis model to control for the potential effect of confounding. Univariate analysis with the chi-square test (Table 2) was used to analyze the association of cervical lymph node metastases with sex, age, tumor size, multifocal tumors, thyroid capsule invasion, vascular invasion, and infiltration of surrounding soft tissues. The results showed that patients with a tumor size of ≥1 cm, multifocal tumors, and thyroid capsule invasion had a higher incidence of cervical lymph node metastases. Multivariate regression analyses showed that only thyroid invasion remained significantly associated with cervical lymph node metastases. Tables 3 and 4 show that thyroid capsule invasion was an independent predictive factor for central compartment metastases (p < 0.001; odds ratio, 11.080) and lateral neck metastases (p < 0.001; odds ratio, 9.067). In our cohort, a preoperative CEA level of ≥30 ng/mL was associated with a higher incidence of central cervical lymph node metastases than was a CEA level of <30 ng/mL (60.0% vs. 34.3%, respectively; χ2 = 4.298; p = 0.038). Receiver operating characteristic curves were used to assess the serum CEA level as a predictor of cervical lymph node metastasis of MTC. As shown in Figure 1, the area under the curve was 0.720 (95% confidence interval, 0.592–0.847; p = 0.002).
Table 2.
Univariate analyses of lymph node metastases in patients with sporadic medullary thyroid carcinoma.
| Factors | Total |
Central compartment metastases |
Lateral compartment metastases |
||||
|---|---|---|---|---|---|---|---|
| n (%) | χ2 | pa | n (%) | χ2 | pa | ||
| Sex | |||||||
| Male | 27 | 16 (59.3) | 3.192 | 0.074 | 14 (51.9) | 2.703 | 0.100 |
| Female | 38 | 14 (36.8) | 12 (31.6) | ||||
| Age, years | |||||||
| ≥45 | 45 | 21 (46.7) | 0.015 | 0.901 | 19 (42.2) | 0.301 | 0.583 |
| <45 | 20 | 9 (45.0) | 7 (35.0) | ||||
| Tumor size | |||||||
| ≥1 cm | 45 | 24 (53.3) | 3.859 | 0.049 | 23 (51.1) | 8.547 | 0.003 |
| <1 cm | 20 | 6 (20.0) | 3 (15.0) | ||||
| Multifocality | |||||||
| Yes | 17 | 13 (76.4) | 8.514 | 0.004 | 11 (64.7) | 5.855 | 0.016 |
| No | 48 | 17 (35.4) | 15 (31.3) | ||||
| Vascular invasion | |||||||
| Yes | 22 | 8 (36.4) | 1.283 | 0.257 | 8 (36.4) | 2.637 | 0.104 |
| No | 43 | 22 (51.2) | 18 (41.9) | ||||
| Capsule invasion | |||||||
| Yes | 26 | 21 (80.8) | 20.893 | <0.001 | 20 (76.9) | 19.752 | <0.001 |
| No | 39 | 9 (25.0) | 6 (15.4) | ||||
aResults of chi-square test between lymph node involvement and tumor characteristics.
Table 3.
Multivariate logistic regression analyses for central compartment metastases in patients with sporadic medullary thyroid carcinoma.
| Factors | β | SE | Wald | pa | OR | 95% CI |
|---|---|---|---|---|---|---|
| Tumor size | 0.076 | 0.648 | 1.433 | 0.231 | 2.173 | 0.610–7.744 |
| Multifocality | 1.461 | 0.782 | 3.494 | 0.062 | 4.311 | 0.931–19.949 |
| Capsule invasion | 2.405 | 0.658 | 13.372 | <0.001 | 11.080 | 3.053–40.214 |
aAccording to multivariate logistic regression analyses. SE, standard error; OR, odds ratio; CI, confidence interval
Table 4.
Multivariate logistic regression analyses for lateral compartment metastases in patients with sporadic medullary thyroid carcinoma.
| Factors | β | SE | Wald | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| Tumor size | 1.023 | 0.674 | 2.300 | 0.129 | 2.780 | 0.742–10.426 |
| Multifocality | 0.824 | 0.745 | 1.222 | 0.267 | 2.280 | 0.529–9.827 |
| Capsule invasion | 2.263 | 0.635 | 12.682 | <0.001 | 9.607 | 2.766–33.374 |
SE, standard error; OR, odds ratio; CI, confidence interval
Figure 1.
Receiver operating characteristic curve of preoperative carcinoembryonic antigen levels predicting cervical lymph node metastases in patients with sporadic medullary thyroid carcinoma.
Discussion
Neck lymph node metastases are reportedly the main predictor of local recurrence and distant metastases in patients with MTC, mainly in the lungs but also in the liver and bones.11–14 This indicates that the dissection of metastatic lymph nodes in patients with MTC, if detected and removed completely during the initial surgery, may prevent reoperation for recurrence in the neck. However, accurate estimation of cervical lymph node involvement is largely dependent upon preoperative imaging examination or intraoperative assessment, which are insufficient. According to a survey by Moley and DeBenedetti,15 intraoperative lymph node assessment by surgeons will miss one-third of clinically involved lymph nodes. Thus, prophylactic neck lymph node dissection provides the best possibility of achieving curative surgery for some clinically node-negative patients.
Based on the 2015 American Thyroid Association (ATA) and 2014 British Thyroid Association (BTA) guidelines, standard treatment for MTC should include total thyroidectomy and central lymph node dissection; however, the extent of dissection in the lateral neck compartments and on the side opposite the largest primary thyroid tumor remains controversial.9,12–14,16,17 The ATA guideline recommends that patients with MTC confined to cervical lymph node metastases should undergo dissection of the involved lateral neck compartments (levels II–V), whereas the BTA guideline recommends that prophylactic lateral neck dissection should be performed in the presence of central compartment node metastases.9,16,18 The need for prophylactic bilateral lateral compartment node dissection in the presence of central compartment node metastases is still unclear. The ATA suggests using the serum calcitonin level as a reference, but this recommendation does not seem to have been widely accepted because of the uncertainness of patient survival.9
Considering this background, a thorough understanding of the clinical risk factors is needed to identify high-risk patients requiring prophylactic lateral neck lymph node dissection. Esfandiari et al.19 reviewed data from 2968 patients with MTC from the National Cancer Database and found a close relationship between tumor size and the likelihood of lymph node metastases; the authors thus suggested that surgeons should decide to perform prophylactic lateral neck lymph node dissection based on the tumor size. Machens et al.11 found that approximately 70% of patients with central compartment node metastases had lateral compartment node metastases. This finding demonstrates that prophylactic dissection of the ipsilateral neck compartment is necessary for patients with MTC with one positive central lymph node, and the performance of contralateral neck lymph node dissection should depend on histopathologic confirmation of lymph node metastases obtained on fresh frozen section.11,20
Our retrospective study of 65 consecutive patients with SMTC treated by total thyroidectomy and central or lateral neck dissection confirms that lymph node metastasis is frequent (found in 52.3% of patients in our study). This finding is consistent with other series: cervical lymph node metastases in patients with MTC have been reported to occur in 25% to 63% of patients, whether the primary tumors are <1 cm or >4 cm.15,21 The univariate analysis in the present study showed that cervical lymph node metastases spread frequently in patients with a tumor size of >1 cm, multifocal tumors, and thyroid capsule invasion, while the multivariate logistic regression analysis demonstrated that only thyroid capsule invasion was an independent predictive factor for cervical compartment metastases. However, in their evaluation of 2968 patients with MTC, Esfandiari et al.19 found that the percent of metastatic cervical lymph nodes was 43% in patients with a tumor size of <2 cm and 65% in those with a tumor size of >2 cm, indicating a relationship between tumor size and cervical lymph node metastasis. In the present study, the univariate analysis showed that cervical lymph node metastases spread frequently in patients with a tumor size of >1 cm; however, the multivariate logistic regression analysis revealed that tumor size was not an independent predictive factor for cervical compartment metastases. This difference might have been due to our classification of tumor size or our smaller sample cohort. A close multivariate correlation between thyroid capsule invasion and lymph node metastases in patients with MTC was demonstrated, which is consistent with the result reported by Machens et al.22 This clearly demonstrates that prophylactic lateral neck dissection should be performed during the initial procedure in patients with SMTC with thyroid capsule invasion. From a clinical perspective, this may help to resolve some of the controversy surrounding the indication for prophylactic lateral neck dissection.
Calcitonin and CEA levels are fairly powerful biomarkers with which to confirm the diagnosis of MTC.6,9 According to previous studies, a serum CEA level of ≥30 ng/mL is suggestive of lymph node metastases in the central and lateral neck compartments.1,7,23 In the present study, we also found that higher stratified basal CEA serum levels reflected the presence of cervical lymph node metastases. Unfortunately, the calcitonin level was not recorded in our whole cohort; therefore, the doubling time of calcitonin, which is known to be a better predictor of cervical lymph node metastases, could not be calculated. This may be the main limitation of our study. Another inherent limitation of this study is that it was retrospective. Unlike in randomized controlled trials, there is also a risk for treatment selection bias.
Conclusion
This study may help to resolve some of the controversy surrounding the indication for lateral lymph node dissection. Prophylactic lateral neck dissection should be performed during the initial procedure in patients with thyroid capsule invasion or a high serum CEA level.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Dralle H andMachens A.. Lymph node dissection in thyroid cancer In: Hubbard J., Inabnet W., Lo CY. (eds) Endocrine Surgery. London: Springer, 2009, pp. 173–193. DOI: 10.1007/978-1-84628-881-4_13 [Google Scholar]
- 2.Machens A, Niccoli-Sire P, Hoegel J, et al. Early malignant progression of hereditary medullary thyroid cancer. N Engl J Med 2003; 349: 1517–1525. [DOI] [PubMed] [Google Scholar]
- 3.Raue F andFrank-Raue K.. Long-term follow-up in medullary thyroid carcinoma. Recent Results Cancer Res 2015; 204: 207–225. [DOI] [PubMed] [Google Scholar]
- 4.Accardo G, Conzo G, Esposito D, et al. Genetics of medullary thyroid cancer: an overview. Int J Surg 2017; 41: 370–373. [DOI] [PubMed] [Google Scholar]
- 5.Machens A andDralle H.. Peak calcitonin cut‐off levels for the diagnosis of occult medullary thyroid cancer: evidence of confounding by gender. Clin Endocrinol (Oxf) 2010; 73: 274. [DOI] [PubMed] [Google Scholar]
- 6.Barbet J, Campion L, Kraeber-Bodéré F, et al. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocr Metab 2005; 90: 6077–6084. [DOI] [PubMed] [Google Scholar]
- 7.Maia AL, Siqueira DR, Kulcsar MA, et al. Diagnosis, treatment, and follow-up of medullary thyroid carcinoma: recommendations by the Thyroid Department of the Brazilian Society of Endocrinology and Metabolism. Arq Bras Endocrinol 2014; 58: 667–700. [DOI] [PubMed] [Google Scholar]
- 8.Kebebew E, Ituarte PH, Siperstein AE, et al. Medullary thyroid carcinoma. Cancer 2000; 88: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 9.Wells SA, Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma: the American Thyroid Association Guidelines Task Force on medullary thyroid carcinoma. Thyroid 2015; 25: 567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konstantinidis A, Stang M, Roman SA, et al. Surgical management of medullary thyroid carcinoma. Updates Surg 2017; 69: 151–160. [DOI] [PubMed] [Google Scholar]
- 11.Machens A Hauptmann S andDralle H.. Prediction of lateral lymph node metastases in medullary thyroid cancer. Br J Surg 2008; 95: 586–591. [DOI] [PubMed] [Google Scholar]
- 12.Scollo C, Baudin E, Travagli JP, et al. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocr Metab 2003; 88: 2070–2075. [DOI] [PubMed] [Google Scholar]
- 13.Weber T, Schilling T, Frank-Raue K, et al. Impact of modified radical neck dissection on biochemical cure in medullary thyroid carcinomas. Surgery 2001; 130: 1044–1049. [DOI] [PubMed] [Google Scholar]
- 14.Jin LX andMoley JF.. Surgery for lymph node metastases of medullary thyroid carcinoma: a review. Cancer 2016; 122: 358–366. [DOI] [PubMed] [Google Scholar]
- 15.Moley JF andDeBenedetti MK.. Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg 1999; 229: 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014; 81: 1–122. [DOI] [PubMed] [Google Scholar]
- 17.Conzo G, Docimo G, Pasquali D, et al. Predictive value of nodal metastases on local recurrence in the management of differentiated thyroid cancer. Retrospective clinical study. BMC Surg 2013; 13: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pena I, Clayman GL, Grubbs EG, et al. Management of the lateral neck compartment in patients with sporadic medullary thyroid cancer. Head Neck 2018; 40: 79–85. [DOI] [PubMed] [Google Scholar]
- 19.Esfandiari NH, Hughes DT, Yin H, et al. The effect of extent of surgery and number of lymph node metastases on overall survival in patients with medullary thyroid cancer. J Clin Endocr Metab 2013; 99: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machens A Holzhausen H-J andDralle H.. Contralateral cervical and mediastinal lymph node metastasis in medullary thyroid cancer: systemic disease? Surgery 2006; 139: 28–32. [DOI] [PubMed] [Google Scholar]
- 21.Rougier P, Parmentier C, Laplanche A, et al. Medullary thyroid carcinoma: prognostic factors and treatment. Int J Radiat Onco 1983; 9: 161–169. [DOI] [PubMed] [Google Scholar]
- 22.Machens A, Holzhausen HJ, Lautenschläger C, et al. Enhancement of lymph node metastasis and distant metastasis of thyroid carcinoma. Cancer 2003; 98: 712–719. [DOI] [PubMed] [Google Scholar]
- 23.Machens A andDralle H.. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocr Metab 2010; 95: 2655–2663. [DOI] [PubMed] [Google Scholar]