Fig. 8.
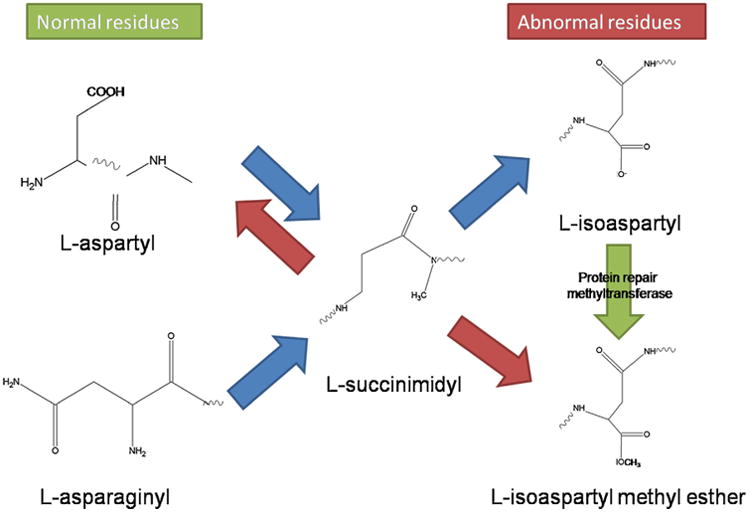
Pathways of non-enzymatic chemical degradation of aspartyl and asparaginyl residues in proteins and of the methyltransferase-mediated repair mechanism. Spontaneous degradation of normal l-aspartyl and l-asparaginyl residues lead to the formation of a ring succinimidyl intermediate. This can spontaneously hydrolyze to either the l-aspartyl residue or the abnormal l-isoaspartyl residue. The l-isoaspartyl residue is specifically recognized by the protein l-isoaspartate (d-aspartate) O-methyltransferase. The result is the formation of an unstable methyl ester that is converted back to l-succinimidyl. Net repair occurs when the l-succinimidyl residue is hydrolyzed to the l-aspartyl form. With the exception of the repair methyltransferase step, all the reactions are non-enzymatic. Adapted from (Clarke, 2003).
