Abstract
Geographic information systems are being increasingly used to show the distributions of disease where data for specific environmental risk factors are available. For successful transmission of schistosomiasis, suitable climatic conditions and biological events must coincide; hence its distribution and prevalence are greatly influenced by environmental factors affecting the population of snail intermediate hosts and human hosts. Prevalence and demographic data was obtained by parasitological examination of urine samples and questionnaire administration. The mean values of environmental factors corresponding to the local government area were obtained from remotely sensed images and data from climate research unit. The effects of the environmental factors were determined by using regression analysis to analyse the correlation of environmental factors to prevalence of schistosomiasis. There was a negative correlation between infection and elevation. There was a positive correlation between vegetation, rainfall, slope, temperature and prevalence of infection. There was also a weak negative correlation between proximity to water body and prevalence. The result shows the study area to be at low to high risk of infection.
Keywords: Correlation, Environmental Factors, Gis, Prevalence, Risk, Schistosomiasis
1. Introduction
Schistosomiasis is a major health problem in Africa. Urinary schistosomiasis is endemic in 44 African countries including Nigeria with an estimated population of 101.28 million people who are at risk with 25.83 million persons infected (Vinod, 2008).
Despite the mass chemotherapy aimed at reducing morbidity, the prevalence of the disease in Nigeria may be increasing due to poverty, inadequate or total lack of public health facility, low illiteracy level and inadequate infrastructure. The lack of scientific information on the disease in many rural communities among the high risk groups particularly school age children is another important factor that has adversely affected control efforts.
The distribution patterns of parasitic diseases are greatly influenced by environmental factors. Geographic information systems and remote sensing have become important tools in predicting infection risk and identifying environment factors at local and broad scale related to risk and therefore allows decision makers to allocate limited resources meant for control interventions in a cost effective manner (Brooker et al., 2002a, Yang et al., 2005). Several studies have developed Africa wide risk maps for the transmission of malaria using climatic determinants of parasites transmission (Simoonga et al., 2009). To date, for schistosomiasis no such maps except for several ongoing projects aimed at achieving this. One of the challenges in developing a continent-wide environmental based schistosomiasis risk map is the variation in biotic requirement of different snail host species that can be found all over Africa. It has been found that climatic suitability risk maps are peculiar for a particular location and cannot be used to predict risk in other places. However, each model could be extrapolated within the same ecological zone presumed to reflect the distribution of the different host snail species. (Brooker et al., 2002b). There is a need to develop an integrated risk map for schistosomiasis that takes into account variations in different climatic zones in order to develop an Africa wide risk map for schistosomiasis. Thus far, in Nigeria, a model of schistosomiasis has been developed at the national scale by Ekpo et al. (2013) and few other models at local scales (Ekpo et al., 2008, Adie et al., 2014). The present report appears to be the first attempt at developing a schistosomiasis risk map for Ondo State albeit at a local scale.
2. Methodology
2.1. Study area
Ondo State is situated in the heartland of the tropical rainforest belt of Western Nigeria. The climate is humid with small seasonal and daily variations. It lies between latitude 5°6N and 8°2N and longitude 4°17N and 6°17N. The average rainfall is concentrated during the months of May to October with a short break in August and considerable variations from year to year. Ile Oluji/Oke Igbo is one of the local government areas in Ondo state.
2.2. Field survey
The current prevalence of disease was determined by parasitological analysis of urine samples collected from inhabitants of geocoded buildings. Infections were detected by demonstration of eggs in urine. Questionnaires were also administered to obtain demographic and socio economic characteristics of the study population.
2.3. Remote sensed image and environmental data
Land surface temperature (LST) and the normalized difference vegetation index (NDVI) and altitude information were derived from satellite images using standard procedures. Minimum, mean and maximum values of these data were extracted for each pixel that corresponds to the location of the study area. Rainfall data was obtained from climate research units.
2.4. Data analysis
Analysis of relationships between household infection prevalence, environmental data and location of water bodies was studied using appropriate software (ESRI ARCGIS 10) Spatial Statistics and regression analyses were used to analyse the correlation of environmental factors to prevalence of schistosomiasis and develop a risk map in correlation to transmission risk.
3. Results
3.1. Prevalence based on households
The results reveal that out of the 526 households screened, 123 households (23.4%) harboured at least one individual positive for schistosomiasis infection (Table 1, Table 2, Table 3).
Table 1.
Prevalence of S. haematobium infection in relation to gender (P < 0.05).
| Sex | No examined | No (%) positive | No (%) negative |
|---|---|---|---|
| M | 356 | 76(21.3)a | 280(78.7) |
| F | 404 | 50(12.4)b | 354(87.6) |
| Total | 760 | 126(16.6) | 634(83.4) |
Table 2.
Prevalence of S. haematobium infection in relation to age (P < 0.05).
| Age | No examined | No (%) positive | No (%) negative |
|---|---|---|---|
| < 10 | 200 | 33(16.5)a | 167(83.5) |
| 10–19 | 251 | 59(23.5)b | 192(76.5) |
| 20–29 | 102 | 24(23.5)c | 78(76.5) |
| 30–39 | 76 | 6(7.9)d | 70(92.1) |
| 40–49 | 55 | 3(5.5)e | 52(94.5) |
| 50–59 | 33 | 1(3.0)f | 32(97.0) |
| > 59 | 43 | 0(0.0)g | 43(100.0) |
| Total | 760 | 126(16.6) | 634(83.4) |
Table 3.
Prevalence of S. haematobium infection in relation to occupation (P < 0.05).
| Occupation | No examined | No (%) positive | No (%) negative |
|---|---|---|---|
| Student | 492 | 101(20.5)a | 391(79.5) |
| Fisherman | 4 | 1(25.0)b | 3(75.0) |
| Farmer | 45 | 3(6.7)c | 42(93.3) |
| Trader | 120 | 7(5.8)d | 113(94.2) |
| Skilled labour | 54 | 6(11.1)e | 48(88.9) |
| Civil servant | 25 | 7(28.0)f | 18(72.0) |
| Professional | 7 | 0(0.0)g | 7(100.0) |
| Others | 13 | 1(7.7)h | 12(92.3) |
| Total | 760 | 126(16.6) | 634(83.4) |
The environmental variables analysed include elevation, land use, NDVI, rainfall, slope and temperature.
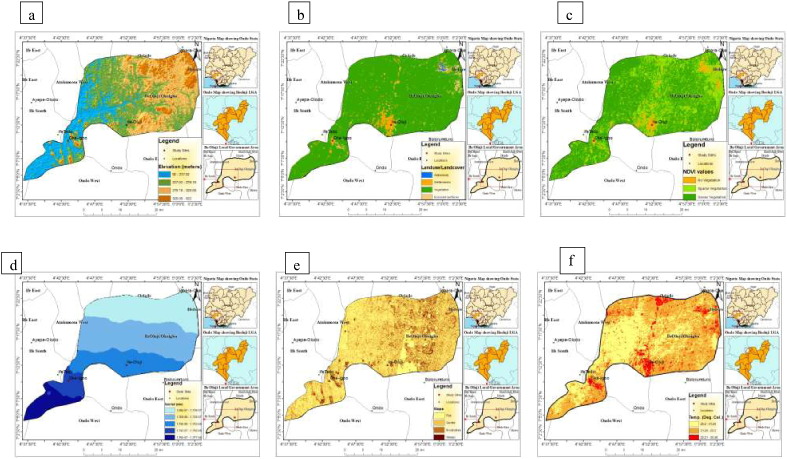
The elevation of the study area was grouped into four levels in metres ranging from 92 to 237.02, 237.03–279.18, 279.19–328.08 and 328.09–522. (Fig. 1a).
Fig. 1.
Map of (a) elevation, (b) landuse, (c) NDVI, (d) rainfall (e) slope, (f) temperature.
The land use of the study area was categorized into four classes: water body, settlements, vegetation and exposed surface. (Fig. 1b).
The vegetation was grouped into three categories: No vegetation, sparse vegetation and dense vegetation. (Fig. 1c).
The annual rainfall (mm) for the period May 2014 to November 2014 was categorized into the levels ranging from 1692.67–1709.67, 1709.68–1726.67, 1726.68–1734.66, 1734.67–1760.66 and 1760.67–1,777.66. (Fig. 1d).
The slope of the study area was grouped into four classes, flat, gentle, moderate and steeply. (Fig. 1e).
Land surface temperature was graded into three categories ranging from 20.2c-21.28°C, 21.29–22.2°C and 22.21–25.28°C. (Fig. 1f).
3.2. Correlation of environmental factors to prevalence in the study area
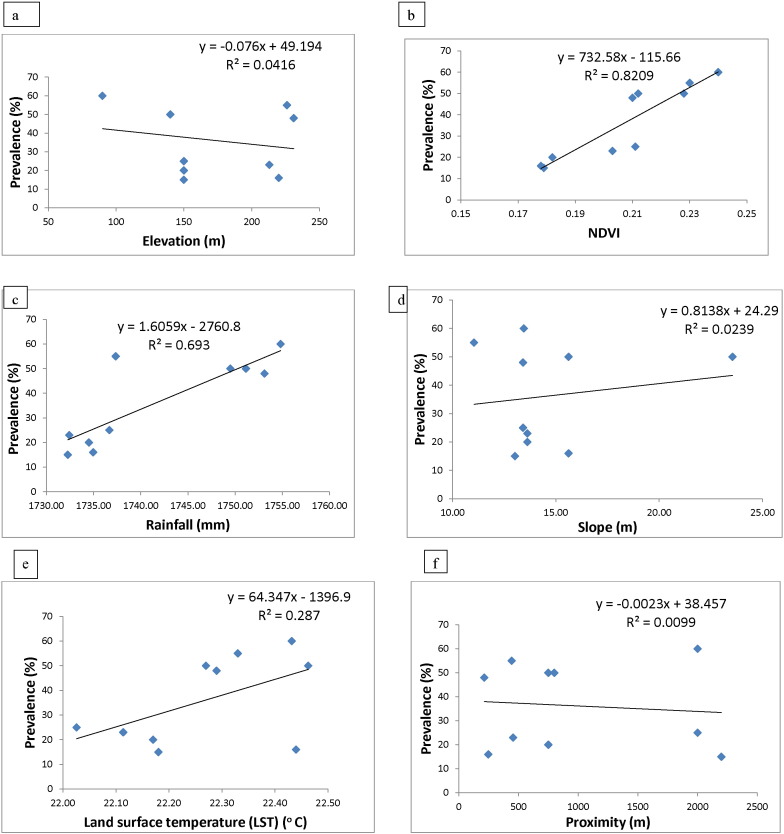
There was a negative correlation between infection and elevation, infection decreased as attitude increase. (Fig. 3a).
Fig. 3.
Graph of (a) elevation, (b) NDVI, (c) rainfall, (d) slope (e) LST, (f) proximity to water body.
There was a positive correlation between vegetation and prevalence. Prevalence increased as the vegetation becomes denser. (Fig. 3b).
Rainfall was positively correlated to infection. Rainfall value increased as prevalence increased. (Fig. 3c).
A positive correlation between prevalence and slope was also observed. (Fig. 3d).
The analysis of the correlation of environmental factors to prevalence of schistosomiasis shows a positive correlation between prevalence and temperature. Infection increased as the mean land surface temperature increased. (Fig. 3e).
Buffers ranging from 100 to 2000 m were created around the river course. There was a positive correlation between proximity to water body and prevalence. Prevalence decreases slowly as proximity to water body increases. (Fig. 3f).
3.3. Risk map of the study area
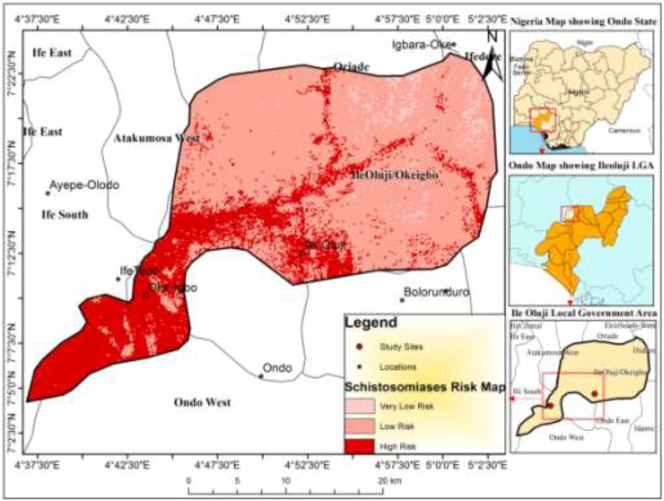
The maps of the environmental variables and prevalence were integrated using a multicriteria analysis to create the risk map. Fig. 2 highlights the spatial distribution of urinary schistosomiasis in the study area.
Fig. 2.
Risk Map for S. haematobium in the study area.
4. Discussion
The results reveal that Oke Igbo harboured more infected individuals than Ile Oluji community. This may be because Oke Igbo has more contaminated body waters and is less developed than Ile Oluji.
The two major environmental factors which are fundamental to schistosomiasis are temperature and rainfall. Both factors show a positive correlation with prevalence data in this present study. The association between temperature and schistosomiasis transmission is expected since the risk of contracting the disease increases during the dry season due to high temperatures, increased water contact either for recreational or domestic purposes and concentration of snails in infected rivers and streams among others (Corina et al., 2006). This is in line with the report by Ekpo et al. (2008) that the significant variable in predicting the absence and presence of urinary schistosomiasis in Ogun state was mean minimum LST. Moodley et al. (2003) who also modelled schistosomiasis in Africa records that maximum and minimum spring and autumn temperatures predicted an increase in the prevalence of Schistosoma haematobium. However, Bavia et al. (1999) reports that annual maximum or minimum temperatures were not shown to be significant factors influencing schistosomiasis prevalence in local populations in Bahia, Brazil. In this study, the high risk area corresponds to areas with temperature > 22 °C, low to moderate risk of infection were found in areas < 22°C. The optimal temperature for snail development and survival is around 25 °C.
An association between snail distribution, abundance and rainfall has been found in several studies. The condition of snail habitat is affected by rainfall in diverse ways. Snails cannot survive without water and too much water also reduces snail population (Simoonga et al., 2009). Rainfall contributes to the creation of temporary snail habitats and also supports creation of new habitats as there is transportation of snails by heavy rainfall. It is also noteworthy that rainfall directly maintains the pollution of water by washing human sewage into potential snail habitats (Corina et al., 2006). The rainfall range that was favourable for snail host populations corresponds with the amount of rainfall in the study area. There was an increase in prevalence as rainfall increases. Rainfall has also been found to be highly correlated with elevation (Raso et al., 2005). It is speculated that both factors have an effect on the flow velocity of rivers which in turn is likely to influence the presence of the intermediate host snail of schistosomiasis. The results of a study by Bavia et al. (1999) however indicate that the duration of the dry season is more significant than the amount of rainfall or length of the wet season in influencing distribution and transmission of Schistosoma mansoni in Bavia. This result supports the analysis from water contact behaviour which shows a high and prolonged water contact during dry season leading to increased transmission, even with a reduction in snail population at this period. The model by Moodley et al. (2003) also supported the hypothesis that rainfall correlates positively with S. haematobium prevalence. Precisely, likelihood of transmission of schistosomiasis in the model increased as annual rainfall increases. However, according to Anderson (1987), the spatial relationship between rainfall and snail population dynamics and infection transmission is difficult to measure since the effect of rainfall varies depending on species of snail and the geographical location.
Elevation determines stream order and flow velocity of rivers which influences the presence of snail hosts. In this study, the prevalence and elevation was negatively correlated. Infection decreased as the attitude increased. This is in agreement with the report by Ekpo et al. (2008) who discussed that there was no relationship between altitude and prevalence of urinary schistosomiasis in Ogun state. This result is however different from investigation in Tanzania and Egypt where attitude is recognized as an important environmental factor in prevalence of urinary schistosomiasis. (Brooker et al., 2001, Malone et al., 1997). From the risk map, the area of high elevation coincides with regions of very low risk.
The effect of slope on the transmission of schistosomiasis is not well documented yet. Studies on snails host vectors of schistosomiasis shows that snails prefer a slope of less than 20 m/km (Birley, 1991). In this study, there was a positive relationship between slope and prevalence. Areas of high risk infection were found on gentle and moderate slopes of 10–40 m/km.
The prevalence of infection in relation to land use cover showed that the areas of high risk were composed of settlements and vegetation. This demonstrates that transmission is linked to landscape where people and disease host vector come together at the same habitat. The sparse to dense vegetation within the study area is characteristic of rural settings or agricultural areas where they practise mainly farming and a high prevalence of infection has been reported severally among farmers.
In this study, NDVI was positively correlated with schistosomiasis prevalence. This is consistent with the environmental condition necessary for the development of the snail host since snails are found in vegetated areas. The vegetation provides surface to crawl and deposit egg masses, contributes to the amount of dissolved oxygen in water and also serves as food (Walz et al., 2015). In Ogun state, NDVI did not show any significant association with the presence of urinary schistosomiasis (Ekpo et al., 2008) whereas in Tanzania and Egypt, NDVI was reported to be a significant environmental variable in schistosomiasis prediction. (Brooker et al., 2001, Malone et al., 1997).
Various studies have shown presence of infection in a place to be strongly associated with proximity to infected water bodies. The comparison between prevalence and proximity to water body in this study shows a weak negative relationship. Prevalence decreases as the distance to the water body increases. This was also observed in schools near Lake Victoria where a positive association exists between prevalence of intestinal schistosomiasis and proximity to the lake shore (Simoonga et al., 2009). Also in Kenya, infection was found to be clustered around infected water bodies (Clennon et al., 2004). The increase in prevalence with increasing closeness to water is due to a corresponding increase in water contact activities for domestic, economic or recreational purposes. A high prevalence of infection has been recorded in school children attending schools situated close to infected water bodies.
The risk map revealed a large area of low to high risk of schistosomiasis infection in about 98% of the study area totalling to an area of 72.5km2 with clusters of significant infection pattern. The risk map also predicted a total of 5,936 households at low risk area of infection and 415 household at high risk areas of infection. The development of risk maps in this research shows that all households present in the study area are still at low to high risk of schistosomiasis infection despite the presence of solar boreholes and annual school children chemotherapy. The map predicts that there are no household in areas of very low risk. This observation is important in future studies on schistosomiasis in order to study schistosomiasis transmission and risk in new settlements. It is also noteworthy that the risk map developed during this study correlates perfectly with the Schistosoma haematobuim risk maps for Nigeria developed by Ekpo et al. (2013) under the CONTRAST project. The map predicted that Ondo State is characterized by moderate risk with the possibility of having high risk communities with prevalence in excess of 50%.
5. Conclusion
The integration of disease, spatial and environmental data offers a robust approach for a better understanding of the epidemiology of schistosomiasis. The maps produced in this study are useful in planning, monitoring and evaluation of schistosomiasis control.
Authors' statement
Authors' contributions: OGA conceived the study; OGA, OPA and OIA designed the methodology and did the implementation; OGA and OIA analysed and interpreted the data. OGA and OIA drafted the manuscript; OPA and OIA critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. OGA and OIA are guarantors of the paper.
Funding
None.
Competing interests
None declared.
Ethical approval
The design of the work has been approved by the Ethical Committee of the Ondo State Ministry of Health, Akure, Ondo state.
Acknowledgements
We thank Mrs. Ogunbiyi for giving us non-financial support and Mr. Adeoba for leading us around the communities that are investigated in this study.
References
- Adie H.A., Okon O.E., Arong G.A. Environmental factors and distribution of urinary schistosomiasis in Cross River state, Nigeria. Int. J. Zool. Res. 2014;10:42–58. [Google Scholar]
- Anderson R.M. Determinants of infection in human schistosomiasis. Baillieres Clin. Trop. Med. Commun. Distrib. 1987;2:279–300. [Google Scholar]
- Bavia M.E., Hale L.F., Maere J.B. Geographic information systems and the environmental risk of schistosomiasis in Baltia, Brazil. Am. Soc. Trop. Med. Hygiene. 1999;60(4):566–572. doi: 10.4269/ajtmh.1999.60.566. [DOI] [PubMed] [Google Scholar]
- Birley M.H. Vol. series No2. PEEM (WHO/FAO/UNEP panel of Experts on Environmental Management for vector control) Secretariat WHO; Geneva, Switzerland: 1991. Guidelines for forecasting the vector some disease implications of water resource development. (PEEM Guidelines). [Google Scholar]
- Brooker S., Hay S.I., Isaac W. Predicting the distribution of urinary schistosomiasis in Tanzania using satellite sensor data. Tropical Med. Int. Health. 2001;6:998–1007. doi: 10.1046/j.1365-3156.2001.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S., Hay S.I., Tchuem Tchuente L.A. Using NOAA-AVHRR data to model helminth distribution in planning disease control in Cameroon, West Africa. Photogramm. Eng. Remote. Sens. 2002;68:175–179. [Google Scholar]
- Brooker S., Hay S.I., Burdy D.A.P. Tools from ecology: useful for evaluating infection risk models? Trends Parasitol. 2002;18:70–74. doi: 10.1016/s1471-4922(01)02223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clennon J.A., King C.H., Muchiru E.M. Spatial patterns of urinary schistosomiasis infection in a highly endemic area of coastal Kenya. Am.J.Trop. Med. Hyg. 2004;70:443–448. [PubMed] [Google Scholar]
- Corina C.F., Ricardo J.P.S., Luciano V.D., Flavia T.M. Ieee. 2006. Remote sensing and geographic information systems for the study of schistosomiasis in the State of Minas Gerais, Brazil; pp. 2436–2439. [Google Scholar]
- Ekpo U.F., Mafiana C.F., Adeofun C.O. Geographical information system and prediction risk maps of urinary schistosomiasis in Ogun state, Nigeria. BMC Infect. Dis. 2008;8:74. doi: 10.1186/1471-2334-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekpo U., Hurliman E., Schur N. Mapping and prediction of schistosomiasis in Nigeria using compiled survey data and Bayesian geospatial modelling. Geospat. Health. 2013;7(2):355–366. doi: 10.4081/gh.2013.92. [DOI] [PubMed] [Google Scholar]
- Malone J.B., Abdel-Rahman M.S., El Bahy M.M. Geographic information system and the distribution of schistosomiasis mansoni in the Nile Delta. Parasitol. Today. 1997;13:112–119. doi: 10.1016/s0169-4758(97)01009-0. [DOI] [PubMed] [Google Scholar]
- Moodley I., Kleinschmidt I., Sharp B., Craig M. Temperature-suitability maps for schistosomiasis in South Africa. Ann. Trop. Med. Parasitol. 2003;97:617–627. doi: 10.1179/000349803225001445. [DOI] [PubMed] [Google Scholar]
- Raso G., Matthys B., N'Goran E.K. Spatial risk prediction and mapping of Schistosoma mansoni infections among school children living in western Cote d'lvoire. Parasitology. 2005;131:97–108. doi: 10.1017/s0031182005007432. [DOI] [PubMed] [Google Scholar]
- Simoonga C., Utizinger J., Brooker S. Remote sensing, geographical information system and spatial analysis for schistosomiasis epidemiology and ecology in Africa. Parasitology. 2009;136:1683–1693. doi: 10.1017/S0031182009006222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod K.D. Schistosomiasis. Medscape J. 2008 (Section 1-11) [Google Scholar]
- Walz Y., Wegmann M., Dech S., Raso G. Risk profiling of schistosomiasis using remote sensing: approaches, challenges and outlook. Parasit. Vectors. 2015;8:163. doi: 10.1186/s13071-015-0732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G.J., Vounatsou P., Zhou X.N. A review of geographic information system and remote sensing with applications to the epidemiology and control of schistosomiasis in China. Acta Trop. 2005;96:117–129. doi: 10.1016/j.actatropica.2005.07.006. [DOI] [PubMed] [Google Scholar]