Abstract
The sodium leak channel NALCN is poorly understood, but is reported as a Na+-permeable, nonselective cation leak channel which regulates resting membrane potential and electrical excitability. Previous work has indicated that NALCN currents can be stimulated by activation of several G protein coupled receptors, including the M3 muscarinic receptor. We undertook a study using voltage clamp electrophysiology to investigate NALCN currents. We compared currents elicited from untransfected control HEK239 cells in response to M3R agonists muscarine or Oxotremorine M to currents elicited from cells transfected with M3R only or the M3R plus NALCN and cDNA encoding accessory proteins UNC-80 and Src. Currents with similar properties were observed in all three groups of cells in response to muscarine agonists, in similar proportions of cells tested, from all three groups of cells. Our findings do not support previous electrophysiological studies suggesting that heterologously expressed NALCN functions as a Na+ leak channel in HEK293 cells. More research will be required to determine the molecular requirements for successful expression of the NALCN channel.
Keywords: NALCN, Patch clamp, HEK293, Muscarinic receptor
Abbreviations: NALCN, sodium leak channel, non-selective; ERS, external recording solution; IRS, internal recording solution; M3R, M3 muscarinic receptor
Highlights
-
•
NALCN (sodium leak channel, non-selective) is a poorly understood ion channel.
-
•
Several reports indicate that NALCN current can be recorded from transfected cells.
-
•
Conflicting reports indicate NALCN currents are simply leaky patch clamp seals.
-
•
We were unable to record currents attributable to NALCN in transfected HEK293 cells.
-
•
Our experiments suggest that NALCN does not form channels in HEK293 cells.
1. Introduction
The NALCN (sodium leak channel, non-selective) gene product is thought to form a voltage-independent, non-selective cation leak channel [1]. Other related proteins include voltage-gated Na+ and Ca2+ channels [1], [2]. NALCN function appears to require an intracellular scaffolding protein, UNC-80, and is facilitated by cytoplasmic Src [3]. NALCN mRNA is highly expressed in neurons of the central nervous system but can also be found in some secretory glands and heart tissue [4]. NALCN is thought to influence resting membrane potential of excitable cells and contribute to tonic depolarization facilitating spontaneous activity in some cell types [5], [6] An essential role for of NALCN is demonstrated in knockout mice, where NALCN−/- mice die within hours of birth [1], [7]. Abnormal function of human NALCN has been linked to ataxia, osmoregulation, and metabolism disorders [8], [9], [10].
Ion currents through NALCN channels have been investigated in neurons and heterologous systems. Activation of tachykinin receptors appears to activate NALCN currents in a G-protein-independent mechanism in neurons [11]. The activation of CaSR, a GPCR that acts as an extracellular Ca2+ sensor, is also thought to stimulate NALCN activity under low [Ca2+]e conditions in cultured neurons [12]. Activation of the M3 muscarinic receptor (M3R), with involvement of activated Src has also been suggested to stimulate NALCN Na+ currents in pancreatic β cells, transfected HEK293 cells and Xenopus oocytes [13]. NALCN currents are resistant to tetrodotoxin (TTX) and Cs+, but have been reported to be blocked by 10 μM Gd3+ and by replacement of extracellular Na+ with N-methyl-d-glutamine (NMDG) [1], [13].
The notion that NALCN is a voltage-independent, leak channel has been challenged [2], [14], [15]. Ren and colleagues [1] indicated that HEK293 cells transfected with NALCN exhibited seals less than 1 GΩ after break-in and attributed this to leakage through NALCN, however others were unable to replicate these leakage currents in transfected HEK293 cells [2]. Moreover Spafford's group [14] used patch clamp to investigate currents from HEK293 cell with poor seals and recorded currents closely resembling those Lu et al. [1] attributed to NALCN. However, Spafford's group also reported that application of Gd3+, replacement of extracellular Na+ with NMDG, and reduction of [Ca2+]e, altered currents from leaky patches to resemble so-called pharmacological blockade of NALCN channels [1]. The conclusion was that currents reported by Lu et al. [1] and others were not attributable to NALCN, but instead to current caused by a weak membrane-glass seal during patch clamp recordings [14].
In this report we set out to investigate the properties of ion currents stimulated by activation of M3R in NALCN transfected HEK293 cells. We recorded ion currents from untransfected HEK293 cells, cells transfected with M3R, and M3R plus NALCN, UNC80 and Src, similar to Swayne et al., [13]. We observed that application of muscarinic agonist to cells elicited a fundamentally similar ion current from each experimental group, suggesting that NALCN expression did not produce a M3R-sensitive ion current in transiently transfected HEK293 cells.
2. Materials and methods
2.1. Cell culture and transfection
Plasmids encoding NALCN/eGFP (in a bicistronic pTracer expression vector, CVM promoter, Invitrogen), UNC80 and Src (in a pcDNA3 vector, Invitrogen) were kindly provided by D. Ren (University of Pennsylvania, Philadelphia). Plasmids encoding eGFP and human M3R (pcDNA3.1 vector) were purchased from Genescript (Piscataway, NJ). All cDNAs were confirmed by sequencing. pcDNA3 and pcDNA3.1 utilize the CMV promoter, as did the pcDNA5 expression vectors of Swayne et al. [13].
HEK 293 cells were grown on 35 mm culture dishes in Dulbeco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and incubated at 37°C in 5% CO2. Cells were grown to ∼80% confluency and transfected using 7.5 μL of Lipofectamine 2000 transfection reagent and 0.75 or 1.5 μg of plasmid. Cells were transfected with plasmids encoding either eGFP alone (0.375 μg), M3R and eGFP (0.375 μg each; hereinafter M3R) or M3R, NALCN, Unc80, and Src (0.375 μg each; hereinafter M3R + NALCN/Unc80/Src) as previously reported [1]. Following overnight incubation, transfected cells were re-plated at low densities onto poly-l-lysine coated glass bottom dishes and allowed to attach to the dish for at least 1 h before electrophysiological experiments.
2.2. Electrophysiology
HEK293 cells were visualized using an Olympus BX51 microscope equipped with DIC optics and epifluorescence. Whole cell voltage clamp experiments were performed at room temperature using a HEKA EPC10 patch clamp amplifier running PatchMaster V2x7.3 or V2x8.0 software. The extracellular recording solution (ERS) was composed of (in mM) 125 NaCl, 5 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 2.5 CaCl2, 10 glucose, 20 HEPES, pH 7.2, 290 mOsM. Electrodes had resistance between 2 and 5MΩ when filled with internal recording solution composed of (in mM) 122 CsMeSO4, 10 EGTA, 0.3 NaGTP, 4 Na2ATP, 10 HEPES, 6 MgCl2, and 14 phosphocreatine, pH 7.2, 285 mOsm.
ERS was perfused through the culture dish at a rate of ∼1 mL/min. The muscarinic agonists muscarine iodide or Oxotremorine M (Oxo-M; Tocris) in ERS were applied at 100 μM for 60 s. Cells were held at −20 mV and current was recorded during depolarizing voltage ramps from −80 mV to +20 mV over 1000 ms.
2.3. Data analysis
Data were analyzed using OriginPro V9.1. Cells that exhibited a 50% increase in current at 20 mV after drug application were considered responsive.
3. Results
Cultures used for experiments were either untransfected, or exhibited a transfection efficiency greater than 50% (by expression of eGFP). For experiments we chose only brightly fluorescent cells that were not contacting other cells, were well attached to the dish, and demonstrated apparent membrane integrity. Additionally, only cells exhibiting seals tighter than 1GΩ after break in were used for experiments. After break in, mean seal resistance for untransfected cells, eGFP cells, M3R cells and M3R + NALCN/Unc80/Src cells were not significantly different (3.17 ± 0.21GΩ, 2.6775 ± 0.5 GΩ 2.45 ± 0.29GΩ, and 2.82 ± 0.31GΩ respectively, p > 0.05 one way ANOVA).
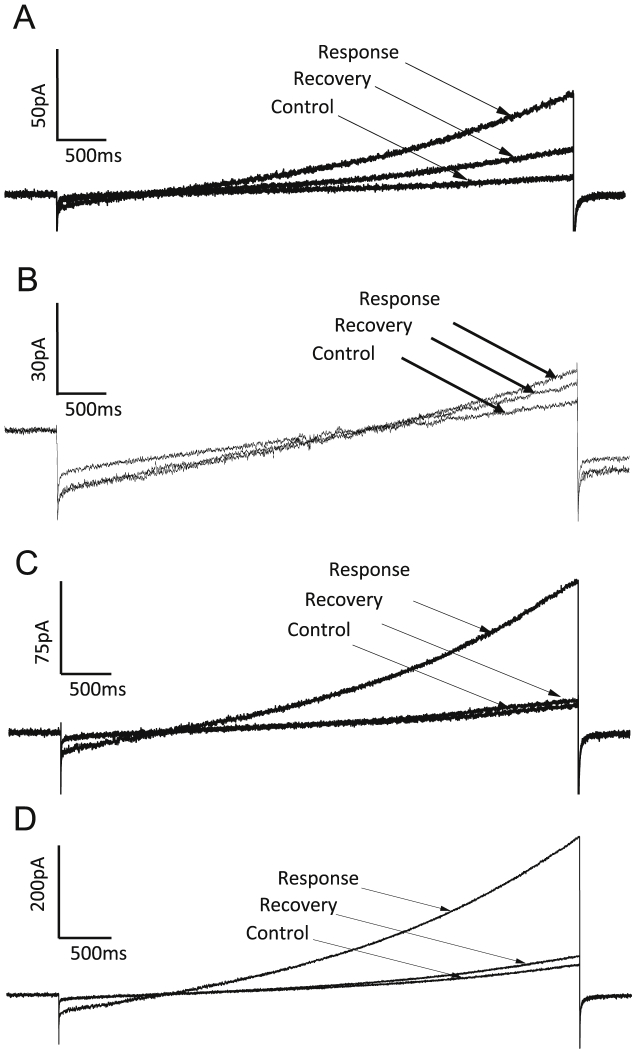
Swayne et al. [13] observed rapidly activating, large inward currents in HEK293 cells transfected with NALCN and M3R in response to application of acetylcholine (ACh). To investigate the currents induced by activation of M3R in HEK293 cells, currents elicited by voltage ramps were recorded before, during and after application of muscarinic agonists to untransfected, eGFP, M3R and M3R + NALCN/Unc80/Src cells. Bath application of agonist increased current at −80 mV and +20 mV in some cells from all three groups, with the current recovering after 3–5 min of washout (Fig. 1A–D). The time course of the ramp currents activated by agonist in all four groups was similar upon visual inspection.
Fig. 1.
Representative currents elicited by voltage ramps (−80 mV to +20 mV over 1000 ms) from (A) untransfected cells, (B) eGFP cells (C) M3R cells, and (D) M3R + NALCN/Unc80/Src cells before application of muscarinic receptor agonist (100 μM muscarine iodide or Oxo-M). Control, response and recovery indicated by arrows.
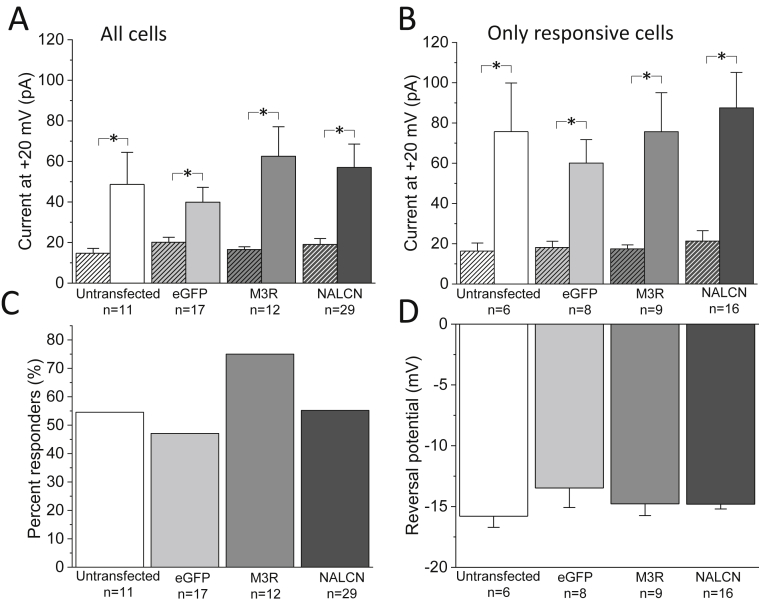
To determine the characteristics of the agonist induced currents, the amplitude at +20 mV of the voltage ramps during response were compared for all three groups using two-way mixed-design ANOVA followed by Bonferroni post hoc tests. We observed significant increases in current at +20 mV following agonist application for each of the groups (within-subject effects) when considering both all cells tested (Fig. 2A) and only the subset deemed responsive (Fig. 2B). Moreover, the mean amplitude of the agonist-induced current was not significantly different between untransfected, eGFP, M3R and M3R + NALCN/Unc80/Src cells (between subject effects, p > 0.05; Fig. 2A and B) We observed that a similar proportion of cells responded to agonist, with 54.6% (6/11) of untransfected cells, 47% (8/17) eGFP cells, 75.0% (9/12) of M3R cells, and 55.2% (16/29) of M3R + NALCN/Unc80/Src cells responding (Fig. 2C; Chi-square, p = 0.51). Additionally, the mean reversal potential of the agonist induced current in untransfected, eGFP, M3R and M3R + NALCN/Unc80/Src cells was −15.8 mV ± 0.9,-13.5 mV ± 1.6, −14.7 mV ± 1.0, and −14.8 mV ± 0.4 respectively (p = 0.27, one way ANOVA; Fig. 2D).
Fig. 2.
Currents observed in M3R and M3R + NALCN/Unc80/Src cells exhibited similar properties to currents observed in untransfected cells and eGFP transfected cells. (A) The amplitude of the current at +20 mV before and after application of muscarinic receptor agonist (muscarine iodide or Oxo-M) at 100 μM for all cells tested. There was a significant main effect between the current amplitude before and after agonist (within-subject effect in a two-way mixed-design ANOVA, DF = 1, p = 7.0 × 10−7, followed by Bonferroni post hoc tests; where * indicates p < 0.05). There were no significant differences between the current amplitudes of untransfected, eGFP, M3R or M3R + NALCN/Unc80/Src cells (between subject effects; DF = 3, p = 0.53). (B) The amplitude of the current at +20 mV before and after application of muscarinic receptor agonist (muscarine iodide or Oxo-M) at 100 μM for only responsive cells. There was a significant main effect between the current amplitude before and after agonist (within-subject effect in a two-way mixed-design ANOVA, DF = 1, p = 9.1 × 10−6, followed by Bonferroni post hoc tests; where * indicates p < 0.05). There were no significant differences between the current amplitudes of untransfected, eGFP, M3R or M3R + NALCN/Unc80/Src cells (between subject effects; DF = 3, p = 0.55). (C) Percentage of untransfected, eGFP, M3R and M3R + NALCN/Unc80/Src cells considered responsive to muscarinic receptor agonist application were not significantly different (p = 0.51, Chi-square test). (D) Mean reversal potentials for currents elicited from responsive untransfected, eGFP, M3R and M3R + NALCN/Unc80/Src cells were not significantly different (p = 0.49, one way ANOVA). All error bars represent ±SEM.
4. Discussion
Swayne et al. [13], reported robust NALCN expression in MIN6 cells, and observed robust inward currents elicited upon application of ACh. Activation of the current was blocked by atropine and an M3R antagonist but not M4R antagonist, and the amplitude of the current was decreased after reduction of NALCN mRNA by lentivirus-mediated RNA interference. Additionally, they demonstrate that NALCN and M3R form a complex in MIN6 cells and transfected HEK293 cells. Swayne et al., also describe currents in HEK293 cells and Xenopus oocytes with properties similar to those observed in MIN6 cells. However in the present study, the currents we observed in response to application of M3R agonists in M3R + NALCN/Unc80/Src HEK293 cells were not significantly different from untransfected, eGFP or M3R transfected cells, either in terms of frequency of cells responding, current amplitude upon activation, or reversal potential. These data indicate that, in our hands, transfection of HEK293 cells with NALCN constructs does not result in a novel cation current as previously published [1], [13].
Senatore el at [2] and Sandstrom [15] were also unable to replicate NALCN currents in HEK293 cells as reported previously [1], [13]. Boone et al. [14] suggested that the reported NALCN currents were the result of a weak membrane glass seal during patch clamp experiments. In this study we set out to investigate the currents induced by M3R activation in M3R and NALCN/Unc80/Src transfected HEK293 cells with the notion that perhaps M3R activation is required. When no significant differences were observed in NALCN-transfected cells from control cells, we concluded that NALCN was not responsible for the observed currents in our experiments, in spite of NALCN RNA expression (confirmed by qPCR, however protein expression not investigated). Instead, we hypothesize that NALCN may not in fact form a functional ion channel when heterologously expressed in HEK293 cells.
HEK293 cells endogenously express M3 muscarinic receptors coupled to phospholipase C pathways that generate inositol 1,4,5 trisphosphate (IP3) and diacylglycerol (DAG) [16]. HEK293 cells have also been found to express a variety of transient receptor potential (TRP) channels including TRPC1, 3, 4, 6, and 7, TRPM 4 and 7, TRPV1, 3, and 5, TRPML1, 2, and 3, and TRPP1 [17], [18], [19]. Of these, TRPC3, 6 and 7 can be activated by DAG and TRPM4 can be activated by intracellular calcium [20]. Activation of these endogenous TRP channels likely explains the currents presently observed in response to muscarinic receptor agonists, although we have not investigated this further. The currents observed are not likely M-currents, as this current is absent from native HEK293 cells [21], [22]. The observation that the agonist induced current was not significantly different between the four cell groups indicates that expression of M3R may not by the limiting factor in determining the proportion of cells expressing the current or the current amplitude.
NALCN is thought to interact with a number of other proteins including UNC80, UNC79, Src, and NLF-1 [1]. While there is little doubt as to a critical role of the NALCN protein in regulating electrical activity of vertebrate and invertebrate neurons, our inability and the inability of others [14] to detect NALCN currents from transfected HEK293 cells indicates that further research is be required to fully understand the complete molecular requirements for successful heterologous expression of NALCN channels. However, NALCN expression is detected in numerous cell systems including MIN6 cells [13] and primary neurons [6], [22], [23], [24], [25]; these are presently the best systems available for study of NALCN properties.
Conflicts of interest
The authors have no conflicts to disclose.
Disclosure
JE and CP carried out the experiments, analyzed the data. WMF and JE co-wrote the manuscript.
Acknowledgments
This work was supported by a Research Manitoba and an NSERC Discovery Grant to WMF, a University of Manitoba GETS award to CP and a University of Manitoba Undergraduate Student Research Award to JE.
Contributor Information
Jennifer M. Egan, Email: eganj3@myumanitoba.ca.
Colleen A. Peterson, Email: petersoc@myumanitoba.ca.
W. Mark Fry, Email: mark.fry@umanitoba.ca.
References
- 1.Lu B., Su Y., Das S., Liu J., Xia J., Ren D. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell. 2007;129:371–383. doi: 10.1016/j.cell.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 2.Senatore A., Spafford J.D. A uniquely adaptable pore is consistent with NALCN being an ion sensor. Channels (Austin) 2013;7:60–68. doi: 10.4161/chan.23981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H., Ren D. UNC80 functions as a scaffold for Src kinases in NALCN channel function. Channels. 2009;3:161–163. doi: 10.4161/chan.3.3.8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senatore A., Monteil A., van Minnen J., Smit A.B., Spafford J.D. NALCN ion channels have alternative selectivity filters resembling calcium channels or sodium channels. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren D. Sodium leak channels in neuronal excitability and rhythmic behaviors. Neuron. 2011;72:899–911. doi: 10.1016/j.neuron.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutas A., Lahmann C., Soumillon M., Yellen G. The leak channel NALCN controls tonic firing and glycolytic sensitivity of substantia nigra pars reticulate neurons. Elife. 2016;5:1689–1699. doi: 10.7554/eLife.15271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y., Abe C., Holloway B.B., Shu S., Kumar N.N., Weaver J.L. Nalcn is a leak sodium channel that regulates excitability of brainstem chemosensory neurons and breathing. J. Neurosci. 2016;36:8174–8187. doi: 10.1523/JNEUROSCI.1096-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinke A.P., Caputo C., Tsaih S.-W., Yuan R., Ren D., Deen P.M.T. Genetic analysis of mouse strains with variable serum sodium concentrations identifies the Nalcn sodium channel as a novel player in osmoregulation. Physiol. Genom. 2011;43:265–270. doi: 10.1152/physiolgenomics.00188.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cochet-Bissuel M., Lory P., Monteil A. The sodium leak channel, NALCN, in health and disease., Front. Cell. Neurosci. 2014;8:132. doi: 10.3389/fncel.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Koh K., Ichinose Y., Yasumura M., Ohtsuka T., Takiyama Y. A de novo mutation in the NALCN gene in an adult patient with cerebellar ataxia associated with intellectual disability and arthrogryposis. Clin. Genet. 2016;90:556–557. doi: 10.1111/cge.12851. [DOI] [PubMed] [Google Scholar]
- 11.Lu B., Su Y., Das S., Wang H., Wang Y., Liu J. Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature. 2009;457:741–744. doi: 10.1038/nature07579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu B., Zhang Q., Wang H., Wang Y., Nakayama M., Ren D. Extracellular calcium controls background current and neuronal excitability via an UNC79-UNC80-NALCN cation channel complex. Neuron. 2010;68:488–499. doi: 10.1016/j.neuron.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swayne L.A., Mezghrani A., Varrault A., Chemin J., Bertrand G., Dalle S. The NALCN ion channel is activated by M3 muscarinic receptors in a pancreatic beta-cell line., EMBO Rep. 2009;10:873–880. doi: 10.1038/embor.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boone A.N., Senatore A., Chemin J., Monteil A., Spafford J.D. Gd3+ and calcium sensitive, sodium leak currents are features of weak membrane-glass seals in patch clamp recordings. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandstrom D. Insight into putative NALCN. PLoS One. 2014 http://journals.plos.org/plosone/article/comment?id=10.1371/annotation/c2983d2f-0c75-4b1c-9274-5ded264b39d9 (Unpublished results) [Google Scholar]
- 16.V Smrcka A., Brown J.H., Holz G.G. Role of phospholipase C in physiological phosphoinositide signaling networks. Cell. Signal. 2012;24:1333–1343. doi: 10.1016/j.cellsig.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zagranichnaya T.K., Wu X., Villereal M.L. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J. Biol. Chem. 2005;280:29559–29569. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- 18.Amarouch M.Y., Syam N., Abriel H. Biochemical, single-channel, whole-cell patch clamp, and pharmacological analyses of endogenous TRPM4 channels in HEK293 cells. Neurosci. Lett. 2013;541:105–110. doi: 10.1016/j.neulet.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Kim W.J., Rivera M.N., Coffman E.J., Haber D.A. The WTX tumor suppressor enhances p53 acetylation by CBP/p300, Mol. Cell. 2012;45:587–597. doi: 10.1016/j.molcel.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Southan C., Sharman J.L., Benson H.E., Faccenda E., Pawson A.J., Alexander S.P.H. The IUPHAR/BPS Guide to Pharmacology in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res. 2016;44:D1054–D1068. doi: 10.1093/nar/gkv1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oldfield S., Hancock J., Mason A., Hobson S.A., Wynick D., Kelly E. Receptor-mediated suppression of potassium currents requires colocalization within lipid rafts. Mol. Pharmacol. 2009;76:1279–1289. doi: 10.1124/mol.109.058008. [DOI] [PubMed] [Google Scholar]
- 22.Yue C., Yaari Y. KCNQ/M channels control spike after depolarization and burst generation in hippocampal neurons. J. Neurosci. 2004;24:4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moose D.L., Haase S.J., Aldrich B.T., Lear B.C. The narrow abdomen ion channel complex is highly stable and persists from development into adult stages to promote behavioral rhythmicity. Front. Cell. Neurosci. 2017;11:159. doi: 10.3389/fncel.2017.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan W.H., Flegal K.M., Chang H.Y., Yeh W.T., Yeh C.J., Lee W.C. Body mass index and obesity-related metabolic disorders in Taiwanese and US whites and blacks: implications for definitions of overweight and obesity for Asians. Am. J. Clin. Nutr. 2004;79:31–39. doi: 10.1093/ajcn/79.1.31. [DOI] [PubMed] [Google Scholar]
- 25.Yeh E., Ng S., Zhang M., Bouhours M., Wang Y., Wang M. A putative cation channel, NCA-1, and a novel protein, UNC-80, transmit neuronal activity in C. elegans. PLoS Biol. 2008;6:e55. doi: 10.1371/journal.pbio.0060055. [DOI] [PMC free article] [PubMed] [Google Scholar]