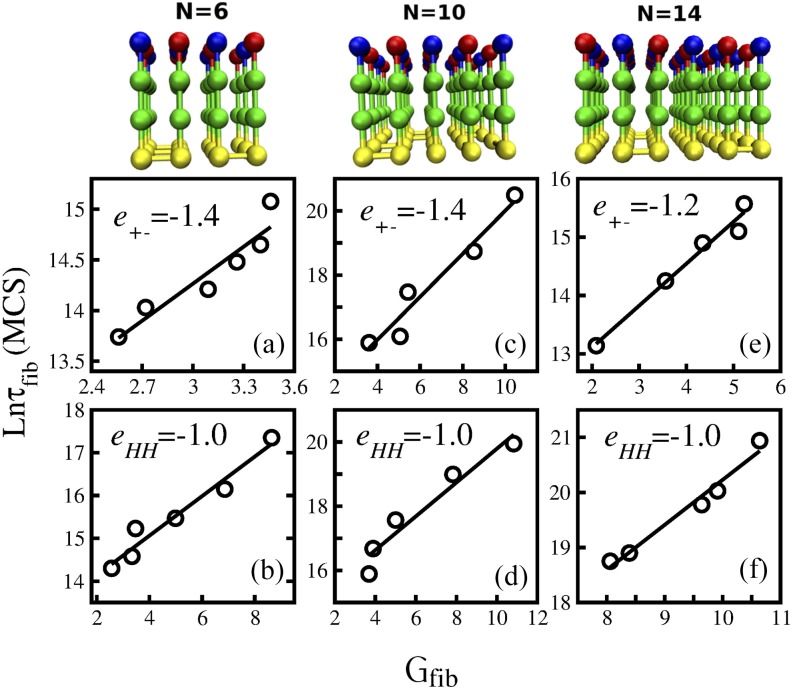
FIG. 2.
Correlation between the formation of polypeptide chains and kinetics stability of the fibril state Gfib. The first row represents fibril structures for N = 6, 10, and 14. The second row shows the dependence of ln τfib on Gfib in the case when the electrostatic interaction e+− is kept fixed but the hydrophobic interaction eHH is varied. The third row is the same as the second row but eHH is fixed, while e+− is varied. (a) N = 6, e+− = −1.4, and the linear fit y = 1.21x + 10.61. (b) N = 6, eHH = −1.0, and y = 0.46x + 13.21. (c) N = 10, e+− = −1.4, and y = 0.67x + 13.3. (d) N = 10, eHH = −1.0, and y = 0.52 + 14.52. (e) N = 14, e+− = −1.2, and y = 0.71x + 11.6. (f) N = 14, eHH = −1.0, and y = 0.81x + 12.0.