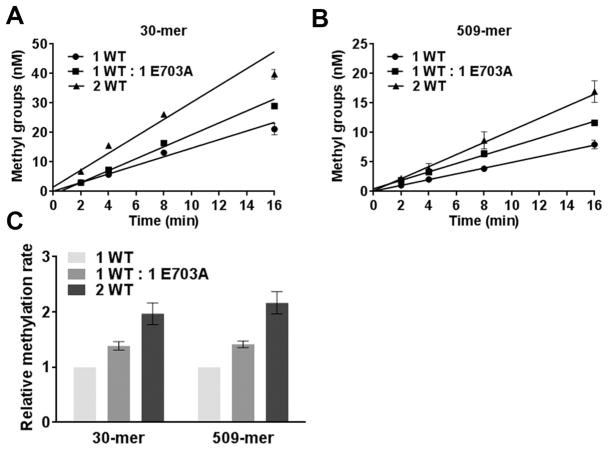
Figure 3.
Dnmt3b-C is not cooperatively stimulated by addition of inactive Dnmt3b-C E703A. (A and B) Methylation kinetics of 30-mer and 509-mer substrates, respectively, by WT (Dnmt3b-C) and a mutant (catalytically inactive Dnmt3b-C E703A). Methylation was conducted using 1 μM WT, 1 μM WT and 1 μM E703A, and 2 μM WT with either 1 μM 30-mer or 150 nM 509-mer substrate. The reaction was initiated by addition of enzyme to the substrate mix, and data were fitted by linear regression, weighted by 1/Y2. (C) Methylation rates were measured from the slopes in panels A and B. The rate of methylation for each substrate was normalized to the rate for 1 μM enzyme and plotted in the bar graph with the normalized error. The average ± the standard error of the mean is shown (n ≥ 4 independent experiments).