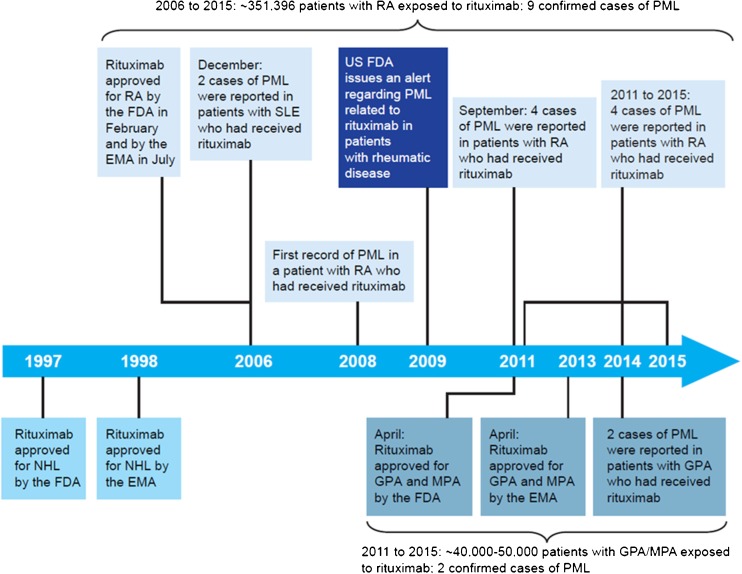
Fig. 1.
History of rituximab and PML in patients with RA or GPA/MPA. References: 2006 SLE cases (Rituxan warning 2007); 2009 RA case (Fleischmann 2009); 2011 RA cases (Clifford et al. 2011); US FDA alert. SLE is not an approved indication for rituximab; two initial cases prompted an FDA warning on PML risk following rituximab treatment. EMA, European Medicines Agency, FDA, Food and Drug Administration, GPA, granulomatosis with polyangiitis, MPA, microscopic polyangiitis, NHL, non-Hodgkin lymphoma, PML, progressive multifocal leukoencephalopathy, RA, rheumatoid arthritis, SLE, systemic lupus erythematosus