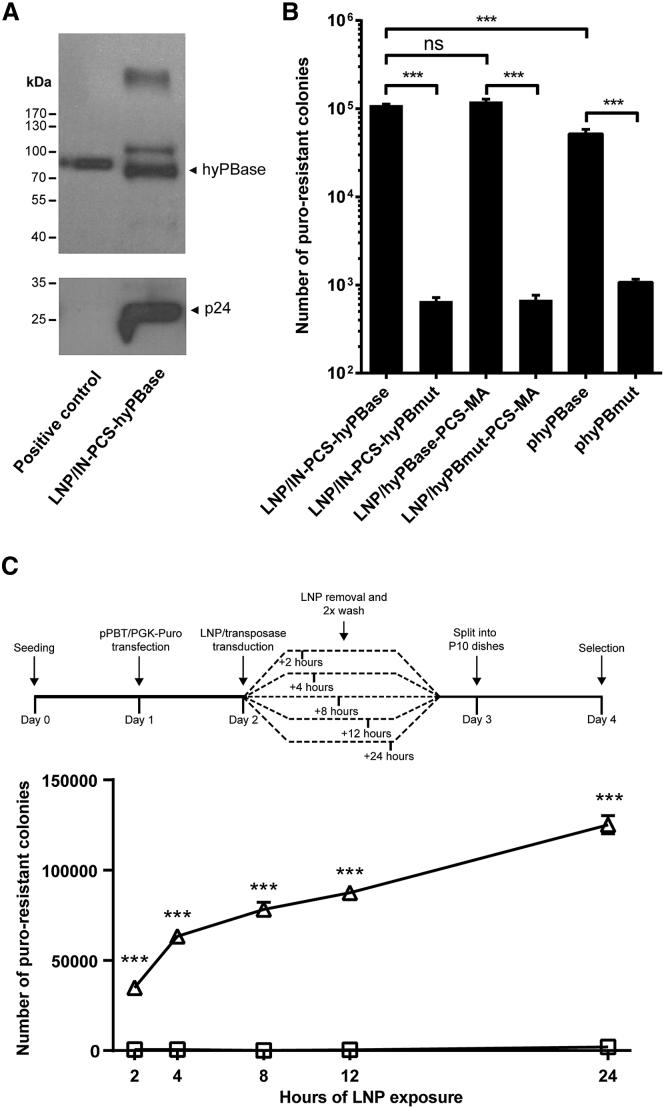
Figure 2.
Efficient and Rapid Cellular Uptake of hyPBase-Loaded LNPs
(A) Evidence of LNP encapsidation of IN-fused hyPBase and subsequent proteolytic release of hyPBase. Western blot analysis on a LNP lysate incorporating an HA-tagged version of the hyPBase transposase is shown. Cell lysates from HEK293T cells transfected with 1 μg HA-hyPBase expressing plasmid served as positive control. (B) Colony formation after transposase delivery by hyPBase-loaded LNPs is shown. Initially, the PB transposon donor pPBT/PGK-Puro was delivered to HeLa cells by plasmid DNA transfection. The cells were subsequently treated with LNPs loaded with either IN- or MA-fused PB transposase, and transposase activity was estimated by quantification of puromycin-resistant colonies. (C) Limited exposure demonstrates rapid cellular uptake of LNPs. HeLa cells were exposed to PB-loaded LNPs for 2, 4, 8, 12, or 24 hr after delivery of the pPBT/PGK-Puro PB transposon donor. Transposase activity was subsequently estimated as described in (B). Triangles, LNP/hyPBase; squares, LNP/hyPBmut. Data are presented as mean ± SEM and n ≥ 3.