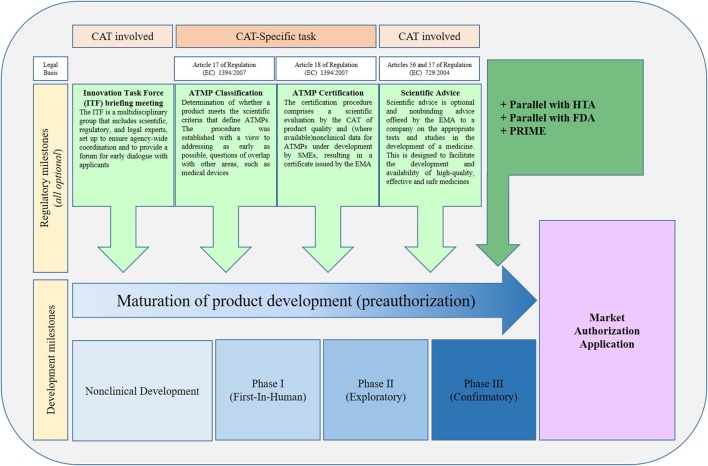
Figure 2.
Regulatory pathways for ATMPs in Europe. The usual sequence in which procedures are requested by applicants. Note that all procedures can be requested at any time during development. ATMP, Advanced Therapy Medicinal Product; CAT, Committee for Advanced Therapies; EMA, European Medicines Agency; FDA, Food and Drug Administration; HTA, Health Technology Assessment body; PRIME, Priority medicine procedures; SME, Small and Medium-sized Enterprise. [Adapted from Maciulaitis et al. (2), (15)].