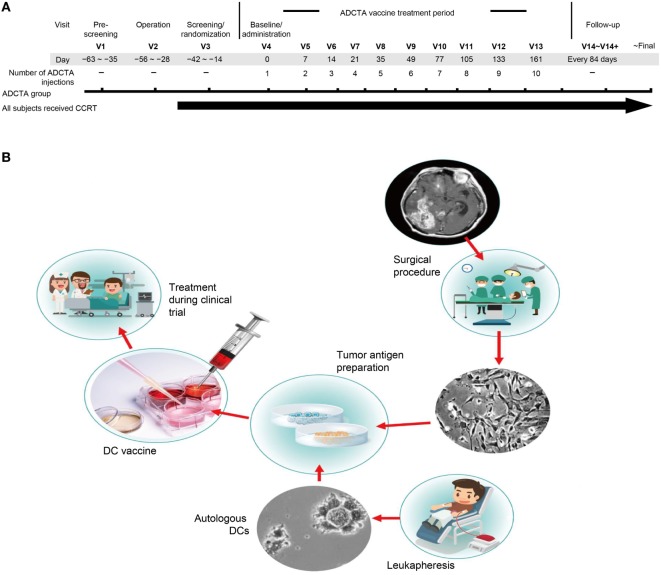
Figure 1.
Treatment schema and vaccine preparation. Clinical schematic diagram (A). Subjects with primary GBM will be consent for operation and concomitant chemoradiotherapy (CCRT). Subjects assigned to the ADCTA group will be designated to receive dendritic cell (DC) vaccination ten times following the clinical trial schedule after operation. V: visits to hospital, numbers following indicates times of visit. DC vaccine manufacturing protocol (B). In China Medical University Hospital, the DC vaccine is produced in laboratories that meet the requirements of Good Tissue Practices and Good Manufacturing Practices. The final product is used in a clinical trial of autologous DC therapy for GBM patients between years 2005 and 2010.