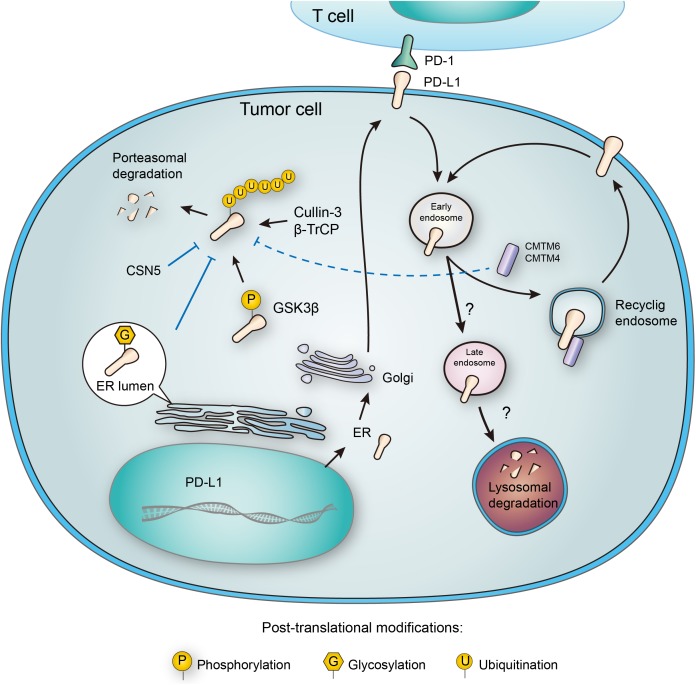
FIGURE 3.
Post-translational modifications and subcellular transportation of PD-L1. As a membrane protein, PD-L1 is extensively modified after its translation. N-glycosylation of PD-L1 extracellular domain occurs in the lumen of endoplasmic reticulum (ER), and this modification facilitates the interaction of PD-L1 with lipid membrane. Glycosylation also inhibits phosphorylation by GSK3β, and thereby blocking the ubiquitination by β-TrCP. Deubiquitination by CSN5 also protects PD-L1 from proteasomal degradation. In addition, PD-L1 may also be destructed in lysosome, and this process relies on a series of subcellular transportations from cell membrane to early endosome, late endosome, and finally to lysosome. However, CMTM6 has been found to promote PD-L1 transportation to recycling endosome, causing decreased distribution to late endosome and lysosome. Interestingly, CMTM6 and its homolog CMTM4 may also stabilize PD-L1 by suppressing its ubiquitination.