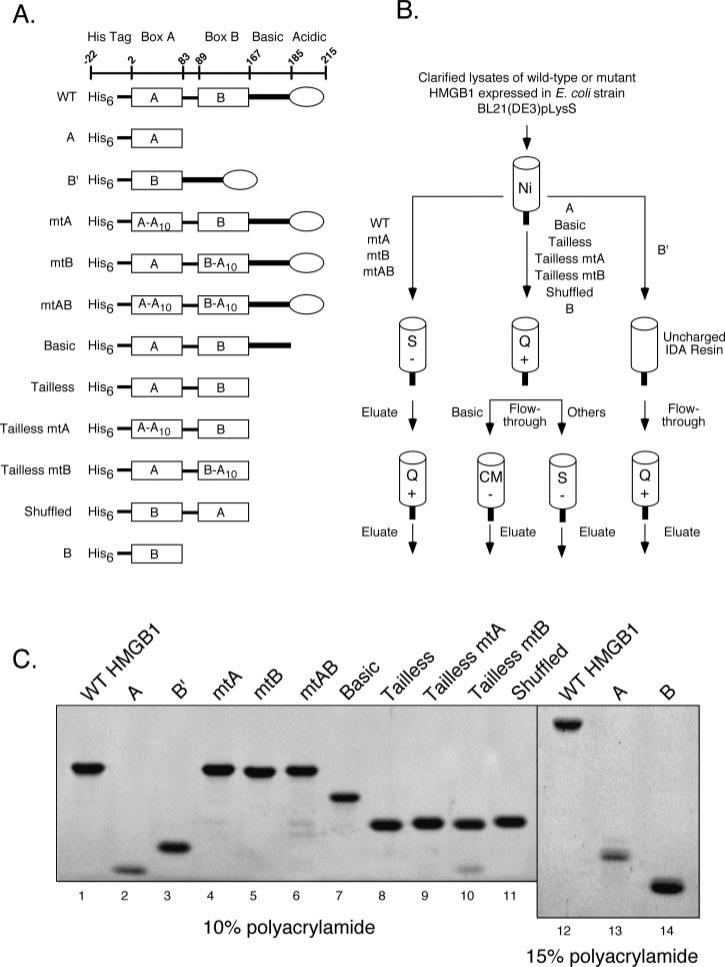
Fig. 1. Purification of HMGB1 proteins.
A, diagrams of HMGB1 proteins used in this study. The amino acid residues encompassing the amino-terminal polyhistidine tag, HMG boxes A and B (rectangles), the basic linker (heavy line), and acidic tail (oval) are indicated at the top. Various wild-type, truncated, and mutant forms of HMGB1 are shown, with designations at the left. Ten consecutive alanine substitutions (A10) have been introduced in either or both HMG-boxes, starting at residue 18 of box A and residue 102 of box B. B, purification strategy of HMGB1 proteins. All proteins were initially purified using IMAC (Ni), with eluates subsequently subjected to chromatography using strongly acidic (S), strongly basic (Q), or weakly acidic (CM) ion exchange supports as indicated. C, purified HMGB1 proteins were fractionated by SDS-PAGE using 10% (lanes 1–11) or 15% (lanes 12–14) polyacrylamide resolving gels, and stained with SYPRO Orange. The identity of purified full-length HMGB1 was confirmed by mass spectroscopy (data not shown).