Abstract
Objective
The purpose of this study was to assess patient perceptions of using an interactive electronic consent (e-consent) application when deciding whether or not to grant broad consent for research use of their identifiable electronic health record (EHR) information.
Materials and Methods
For this qualitative study, we conducted a series of 42 think-aloud interviews with 32 adults. Interview transcripts were coded and analyzed using a modified grounded theory approach.
Results
We identified themes related to patient preferences, reservations, and mixed attitudes toward consenting electronically; low- and high-information-seeking behavior; and an emphasis on reassuring information, such as data protections and prohibitions against sharing data with pharmaceutical companies. Participants expressed interest in the types of information contained in their EHRs, safeguards protecting EHR data, and specifics on studies that might use their EHR data.
Discussion
This study supports the potential value of interactive e-consent applications that allow patients to customize their consent experience. This study also highlights that some people have concerns about e-consent platforms and desire more detailed information about administrative processes and safeguards that protect EHR data used in research.
Conclusion
This study contributes new insights on how e-consent applications could be designed to ensure that patients’ information needs are met when seeking consent for research use of health record information. Also, this study offers a potential electronic approach to meeting the new Common Rule requirement that consent documents contain a “concise and focused” presentation of key information followed by more details.
Keywords: e-consent, broad consent, Common Rule, research informatics, electronic health record, information systems design
BACKGROUND AND SIGNIFICANCE
Electronic health record (EHR) data are increasingly valuable for clinical research. Secondary uses of EHR data include integrating clinical data across facilities and organizations to support quality improvement1–3 and generating more extensive data for research.4,5 Beyond data integration, EHRs increasingly contain new forms of data, such as biospecimens, genomic data, and information on social determinants of health,6–8 which are being used to support individualized or precision health care and related research.9 In addition, EHR systems and data networks are often queried to identify patients who may be eligible for clinical trials.10 Finally, EHR records may contain data more representative of underlying populations,7 thus improving demographic representation in clinical research11–15 and furthering health equity goals. However, the long-term success of research that incorporates EHR data requires support from patients in sharing their health information. Therefore, it is practically and ethically important to ensure that patients are appropriately informed about and agree to research use of their EHR data.
Patients generally agree that prior consent should be obtained for the use of personal health information for research.16–20 For example, recent studies have found that a majority of individuals (>80%17,21) believe that prior consent is necessary for research use of EHR data. In general, as long as permission is obtained and individual privacy is protected, patients are highly supportive of using health information for research purposes.16,17,22,23 In particular, patients tend to trust hospitals and research institutions with their information more than pharmaceutical or insurance companies.16,21,24,25 However, research suggests wide variation in the extent and type of information that patients value when making a consent decision, such as information on privacy and security protections, level of personal control over the use of their data, and purpose of or funding behind the research.17 Patient preferences may also differ around the form of consent (ie, broad, disease-specific, or study-specific) used to obtain their health information.17,21,26,27 In addition, processes for obtaining consent should strive to not impede administrative or clinical workflows while appropriately informing patients.
Understanding how to provide information to patients during consent for research use of EHR data is particularly important in light of recent updates to the US Federal Policy for the Protection of Human Subjects (Common Rule). First, the revised Common Rule, which takes effect in 2018, addresses the length and complexity of typical consent forms by requiring a “concise and focused” presentation of key information. This presentation is then followed by more details. Second, the revised Common Rule gives institutions the option of obtaining broad consent from patients to use individually identifiable health information for future research.28 Using broad consent will be an alternative that investigators can choose in lieu of other options, such as using deidentified information, seeking institutional review board (IRB) waivers of consent, or obtaining study-specific consent.28 Broad consent may be attractive to researchers in cases where a health care organization has implemented an institutional process to consent many patients. Similarly, broad consent may be desirable for patients, because it informs them how their information may be used in research, while also recognizing that most patients do not want to be asked repeatedly about using their information.21,29
Despite recent changes to the Common Rule and well-documented public support for the use of EHR data for clinical research, the question remains: What are best practices for obtaining broad consent in a way that informs patient choice without placing an undue burden on researchers and clinical staff? Some evidence suggests that multimedia applications allow patients to better control the pace of information delivery and may reduce the intensity of staff oversight as compared to paper consent documents.30,31 For example, recent approaches have involved translating standard paper consent forms into videos.32–34 However, previous studies have not focused on patients’ preferences for using an electronic application that allows them to customize the amount and type of information they receive. Furthermore, previous work did not examine patient preferences around the use of electronic tools for broad consent to use their personal health information. Finally, while some research finds that a majority of patients are willing to provide broad consent for biobanks35 and research re-contact,36 little is known about patients’ knowledge, attitudes, and perceptions around broad consent in the context of sharing their EHR data.
OBJECTIVES
The purpose of this study was to assess patient perceptions of using an interactive electronic consent (e-consent) application when deciding whether or not to grant broad consent for research use of their existing and future identifiable health record information. Specifically, we aimed to assess participant attitudes toward and perceptions of (1) using an e-consent application compared to paper, and (2) using an interactive e-consent application in which participants can access on-demand information that goes beyond standard consent requirements. This study informed the design of an e-consent application to be used in a future randomized trial on the effects of different forms of information on patients’ decisions to share their EHR data for research.
METHODS AND MATERIALS
E-consent application development
We designed a prototype e-consent application that asks patients if they are willing to share their existing and future identified health record information for research. The overarching design requirements were that the application: (1) present all Common Rule–required information for a valid informed consent, (2) clearly present information about how research data are safeguarded, and (3) allow users to interactively obtain more detailed information beyond the required information only if they desired. Similar to the requirements for information presentation found in the recent Common Rule updates, we aimed to offer a concise presentation of all key information while also providing a design that allowed users the option of obtaining more detailed information on demand.37 To develop detailed design requirements, we conducted a day-long design and content development workshop with researchers, bioethicists, IRB members, a software designer, and patients. The morning session of the workshop involved 9 people, including a visual designer and research experts in bioethics, health services, informatics, and communication science. The afternoon session involved an additional 5 people who were not part of the core research team, including the local IRB chairperson, another IRB member, and clinical research coordinators familiar with obtaining research consent in primary care practice settings.
The workshop focused on confirming information required for valid broad consent to share EHR information and identifying concepts about which some people might want more detailed information. In terms of interface design, the workshop focused on reviewing and discussing different modes of communicating consent information (eg, text, visualizations, and video) and mechanisms for users to interactively drill down from required consent information to additional details. These additional details provided definitions and examples for key concepts (eg, “health record information,” “password-protected”).
Following the workshop, we synthesized and verified the requirements elicited from the workshop participants to develop an HTML-based application prototype. This process resulted in an application with primarily text-based content, including hyperlinked text that allowed users to click and obtain more detailed information on demand. A total of 19 hyperlinks were added to provide additional information on key terms and concepts related to research processes (see Table 1).
Table 1.
Key concepts and terms from the application associated with informational hyperlinks
| Domain | Hyperlinked concepts and termsa |
|---|---|
| Research processes | “researchers,” “research registry,” “Institutional Review Board” |
| Health records | “health record information,” “information you have shared with any of your doctors or nurses” |
| Risks and benefits of research participation | “not be penalized or lose any benefits to which you are otherwise entitled,” “minimal risk,” “small risk,” “benefit you,” “[risks that] could affect you or your family” |
| Data protections | “kept in a safe location,” “password protected,” “encrypted computer server,” “publish or report your name or other information about you in particular,” “people who should not have [your health information],” “keep your information secure and confidential” |
aSome terms were hyperlinked multiple times, resulting in 19 hyperlinks for 16 unique concepts and terms.
The application consisted of 4 pages that could be navigated sequentially, forward and backward. The first page provided an introduction to the consent task and instructions for using the application. The second page provided a series of key points about how health record data are safeguarded by researchers. The third page provided the required consent details, including research purpose, research benefits, research risks, information privacy, and contact information for asking additional questions. Pages 2 and 3 contained clickable hyperlinks for on-demand information details (Figure 1). The fourth page asked users whether they agreed to allow researchers to access their health record information for research. To provide consent, the application asked users to type their name, sign their name using a touch-based signature pad, type the date, and click a “Yes, I agree” button (Figure 2). After signing the consent document, users were able download and view a PDF copy and take home a paper copy of all content contained in the e-consent.
Figure 1.
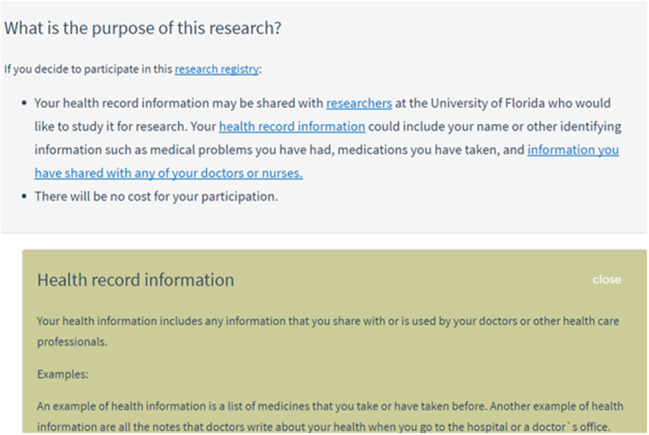
Example of clickable hyperlink to obtain more information on the concept of “health record information”.
Figure 2.
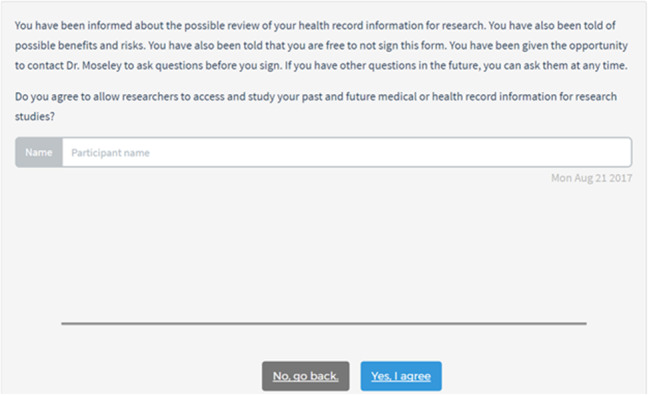
E-consent interface for patients to provide their name and signature.
Setting and participants
We recruited 32 adult participants from a university database of >4000 community members in north-central Florida who had previously agreed to be approached for future research studies. Participants were English-speaking adults (≥18 years old) who reported having at least some experience using computers. All participants received a $25 gift card as remuneration.
Procedures
We conducted think-aloud interviews with individual patients while they used the application. Think-aloud interviews simulate a task while asking users to constantly verbalize their thoughts and decisions and are frequently used in software development, including health care application development.38–41 We conducted interviews in 2 rounds. The first round included 22 participants and was also used to identify design and functionality improvements in the consent application. The second round included an additional 20 interviews that were conducted using the same procedure described above, but with the improved application. We interviewed 10 of the participants from the first round a second time to provide comparative feedback on application improvements; in addition, we interviewed 10 new participants. All interviews were audio-recorded and transcribed.
The 2 interviewers (KR and DH) received 4 hours of interview training led by research team members JK, RM, and CH. The training included information on participants and practice establishing rapport with them, introducing the study scenario, ensuring that participants verbalized their thoughts throughout their use of the e-consent application, and asking probing questions following use of the application. Interviews lasted 60–90 minutes and were conducted by a single interviewer according to a structured protocol (see Supplementary Appendix). First, the interviewer explained to participants that the university was interested in testing a new e-consent application that asked about using health records in research. The interviewer emphasized that the application was still being developed and that researchers were interested in all feedback, including feedback on parts of the application that were confusing and/or needed improvement. Next, the interviewer explained that participants would be asked to constantly verbalize their thoughts and decisions, and led participants through a practice think-aloud task.
To simulate natural use conditions, interviewers asked participants to imagine that they had arrived for a visit to their primary care clinician’s office and had been approached by a staff member to consider consenting to use of their EHR data in research. Interviewers instructed participants to proceed through the application while constantly verbalizing their thoughts, their decisions to seek or ignore information, their decisions to click on interactive features, and any expectations or confusion about the application. During the think-aloud process, interviewers encouraged participants to continue verbalizing their thoughts and asked probing questions to further understand use behavior. After the think-aloud process, the interviewer asked each participant a set of open-ended follow-up questions to evaluate his or her overall satisfaction, perceptions of the application’s usability, understandability of the content, and suggestions for improving the application.
Analysis
We analyzed interview transcripts using a modified grounded theory approach to develop our coding structure.42 Specifically, we developed a set of a priori codes related to topics covered in the interview in conjunction with emergent concepts from participant interviews. First, 2 members of the study team (EG, KR) independently reviewed all 42 interview transcripts and performed open coding. Next, using a provisional set of codes, the 2 team members each coded the same randomly selected subset of 4 transcripts. To assess intercoder reliability, we used Cohen’s kappa (κ) to measure correlation between coded segments while controlling for chance agreement between coders.43 The coding team then met to resolve discrepancies in coding, discuss new codes observed, discuss combining of existing codes, and refine the codebook. We repeated this process until agreement reached κ ≥ 0.80 for the majority of transcripts.44,45 The entire set of transcripts was then split between the 2 team members and coded independently. After coding was completed, we generated reports for each code that included all excerpts to which a code was applied, the frequency of code application, and associations between different codes. We reviewed these code reports and met to identify findings and emergent themes. All data were organized in Dedoose software, version 7 (SocioCultural Research Consultants, Los Angeles, CA, USA).
RESULTS
Table 2 presents descriptive characteristics of participants (N = 32). Participants were, on average, 54 years of age, largely female (88%), and mostly non-Hispanic (94%). In terms of race, 69% were white, 25% were black, and 9% were other races. In terms of education, 31% had a high school diploma or less, 25% had some college, and 44% had a master’s degree or higher.
Table 2.
Descriptive characteristics of participant sample (N = 32)
| Variable | N (Mean) | % (SD) |
|---|---|---|
| Gender | ||
| Female | 28 | 87.5 |
| Male | 4 | 12.5 |
| Age (years) | (54.1) | (14.3) |
| Racea | ||
| White | 22 | 68.8 |
| Black | 8 | 25.0 |
| Other | 3 | 9.4 |
| Ethnicity | ||
| Hispanic | 2 | 6.3 |
| Non-Hispanic | 30 | 93.8 |
| Educational level | ||
| High school or less | 10 | 31.3 |
| Some college | 8 | 25.0 |
| Master’s degree or higher | 14 | 43.8 |
Notes: SD = Standard deviation. aPercentages do not total 100% because an individual participant could select multiple race categories.
Three themes emerged related to attitudes toward providing consent electronically: (1) patient preferences for the electronic consent format, (2) reservations about the electronic format, and (3) mixed attitudes toward the electronic format. Also, 2 themes emerged related to information-seeking: (1) high- vs low-information-seeking behavior, and (2) emphasis on reassuring information. A summary of findings by theme are presented below, along with representative quotes from the interviews.
Attitudes toward consenting electronically
Interviews revealed a range of attitudes among participants toward consenting electronically (Table 3). Although many participants appreciated the modernity of the tablet format, the clarity of the information presented, and the interactivity of the application interface, others had reservations about the use of an electronic consent application. These reservations were commonly tied to a preference for the traditional paper format or concerns about insufficient digital literacy or experience. Still others expressed mixed attitudes toward the application, citing both advantages and disadvantages of a digital format.
Table 3.
Themes related to attitudes toward consenting electronically
| Theme | Representative quotes |
|---|---|
| Preference for electronic format | I: In general, do you think it’d be useful to have electronic? |
| P: In general, yeah. … I mean, a lot of doctors’ offices now have a computer sign-in and all that sort of stuff, you know? | |
| Less paperwork. Who wants paperwork? I’d rather everything paperless. That part’s good. | |
| I could see that this ultimately was gonna be an agreement that I was gonna have to yes, agree to, or not from the very beginning instead of having to go to the very end and not knowing. On pages, hard copy pages, you would normally have to at least go through 4 or 5 pages to realize that there is gonna be an agreement or disagreement on this. That, you could see right off the bat. It came up right away and was always there. | |
| Reservations about electronic format | I’ve never learned how to use a computer, except for I can play Mahjong and Spider Solitaire on it and that’s about it. … I don’t have online service either, so it’s not something I know how to do. |
| This particular type of computer I have in front of me now is not one I usually regularly work with, so there’s a little bit of logistics about learning that. I don’t normally do a touchscreen. I do a mouse. | |
| I would prefer to get something in paper even though it was a lot, because what it allows me to do is more easily refer back. If I’m not sure I understood or something I read, I can always go back, but with electronic it’s not as convenient or as easy. If I had a paper, if I had this paper and a pen in hand, I would probably underline or circle things, important things that I’d want to remember, or think that’s important for me to – in case I need to refer back to it. | |
| Mixed attitudes toward electronic format | I know things can change online. If it’s something I signed – this is a hard copy. You can see what I signed. Sometimes online, things change. I know that I change stuff all the time and stuff, especially if it’s a Google doc or whatever. I guess if it’s e-mailed to me and I had it saved as that, it would say, “Yes, that is exactly what I signed.” Not having the paper around, I think, would be nice. Yeah. I think it’s good. I’m okay with everything digital. I don’t know if some people feel better having paper. |
| Maybe an older person might be a little distrustful of computer. Whereas, when they have a piece of paper, it’s like a legal document. | |
| They might feel more peace of mind when they hold a piece of paper. For more your younger people, you’d be fine, I believe, with an electronic way to do it. |
Participants who favored consenting electronically often cited the general advantages of a modern electronic format. Several participants commented that electronic was simply “the way to go” in many areas of life: “It’s just [that] I think the population is used to digital, and I think it’s really good to use that.” Some of these respondents noted previous experiences seeing tablets or other electronic media used in the context of health care and other settings.
Other respondents remarked on the organizational clarity of the information presented in the application. Many participants thought the electronic version was easier to read, was more concise, and made the information in the consent document more accessible than in a hypothetical paper version; as one respondent noted, “I like how it’s broken up so it’s easier to read. It’s less intimidating upon first glance than a packet of paper.” A handful of participants associated this ease of use with the interactive features (ie, hyperlinks) that allowed for concise content with the option to obtain further information as desired.
Conversely, other participants expressed a clear preference for a paper format. Reasons for this varied, but often included some element of unfamiliarity with computers in general and/or tablet computers specifically. Some respondents who noted past familiarity with desktop computers, smartphones, or other types of tablets described difficulties using the tablet computer for the e-consent application. Some participants cited the familiarity and tangible comfort of the paper form, describing how “some people like paper. They want – if they’re signing a document, they like to read it, and hold it, and keep a copy. … They might feel more peace of mind when they hold a piece of paper.” Several participants remarked that they valued the ability to easily turn back a page or mark information of interest in the document.
Still others expressed mixed feelings about the relative advantages of consenting electronically. Some participants noted that in an ideal setting, electronic consenting might be preferable, but real-world concerns such as usability and security might present obstacles to effective use of an e-consent application in a clinical setting. Many attributed age as a factor in the acceptability of a paper versus an electronic medium. These respondents cited either their own lack of familiarity with electronic devices as an older adult or that of older family members as a potential barrier to use, but felt that younger patients would welcome the application.
Information-seeking behaviors
Participants tended to sort into 2 different groups: those who sought further information on certain keywords or topics using the on-demand hyperlinks (high-information-seeking) and those who did not (low-information-seeking). Furthermore, high-information-seeking participants often focused on similar topics (Table 4). Multiple respondents expressed a lack of knowledge around the meaning of “protected health information” and what kinds of data were included in the EHR (eg, Social Security number, financial information). Also, many respondents had questions related to the details of how their health information would be protected, including more information about the specific meaning of “password-protected” or “encrypted”:
P: “What’s the benefit,” okay. Okay, “how [your data will] be kept private,” oh goodness. “Kept in a very safe location.” I hate qualifiers like that. It doesn’t make me feel very safe.
I: What would make you feel safe?
P: When I see “will be kept in a very safe location,” I would want specifics.
Table 4.
Common topics of interest for high-information-seeking participants
| Common topics | Representative quotes |
|---|---|
| Types of information contained in EHRs | Personal information. I wonder what the personal information is ’cause it seems like anything that would be with my health record is personal information. It seems like, if they wanted to share things about health issues, your sex and your age might be appropriate for when people encounter certain ailments. |
| Purpose of research | Most research groups that I’ve ever gone through have told me, “You are being tested for X. The effects are knowing, or maybe you don’t have them, and what we can do to better serve.” The particular purpose of this research is not very clear. |
| Risks of research participation | That makes me a bit concerned. “However, there is a small risk that a person may see your information without permission.” I would like if someone could call me if that ever happened, especially when it said this could affect you or my family, especially me having a kid. That makes me concerned. |
| Data protections | I would like to – also reassuring would be some reference – some indication in all of this that you’re presenting the consequences for abuse of personal information and how your institution would police that information and how it would detect and identify and sanction anyone who would abuse the information or access it without proper authorization. That would be very reassuring to me as well, and would increase the likelihood that I would give my consent to some form of access to my health care records. |
| Circumstances under which data could be shared | What would be the special circumstances? Why would the government get my information? |
| Research administration processes | I mean, you did a good job showing how we collect the information, what we collect, how it’s used. How it’s stored and kept safe. You mention a PI and you mention an IRB, but you don’t really explain those relationships. |
| Extent and duration of research use of EHR data | I guess I would like to know why, if I’m doing a study, what is the time frame that the researchers will look at my health record information. I know some studies, they wanna look at long term, down the road. That’s my question. I would like that to be clarified. Your past and future medical health record information. That’s a little confusing to me. It doesn’t clarify whether when you’re giving consent that the researchers are only going to look at your past and current medical record information or why they ask about access to your future medical or health record information. |
Respondents commonly expressed a desire to know more about the consequences of risks (ie, “What could happen if my protected health information is compromised?”) and notification in cases where these risks were realized (ie, “If my health information is compromised, how will I be informed?”). Others had questions about the specifics of the research for which they were being asked to consent, such as the topic or purpose of the study: “It would be very helpful to the reader and potential study subject to have some, at least, some examples of the type of research the researchers intend to do.” Some participants commented specifically on the notion of broad consent and wanted to know more about the extent and duration of their control over their personal information.
In contrast, low-information-seeking respondents were content with the amount and detail of information given by the primary consent information:
P: I mean, I really can’t see the purpose in why anybody would wanna sit and ponder this because it – you know, it’s very self-explanatory. I don’t think that you would participate in something like this if you had anything in particular that you didn’t want people to know that would be in your health records.
I: Did you feel that you needed more or less information, and does that have anything to do with why you didn’t click the hyperlinks?
P: Oh, I don’t know. I didn’t think I needed more information.
Both high- and low-information-seeking participants expressed feelings of reassurance around specific statements or explanations provided in the application (Table 5). Commonly, participants described how they were comforted by information demonstrating researcher/institutional expertise, the presence of safeguards around their health data, and prohibitions against sharing their information with pharmaceutical companies. Other topics of reassurance included knowing the name and contact information of the study’s principal investigator, patient rights as a research subject, and the fact that their participation in research would not cost them anything.
Table 5.
Types of information participants found reassuring
| Type of reassuring information | Representative quotes |
|---|---|
| Data protections | The key is set, is kept very safe by the computer and only given to people who are allowed to see your list of medicines. I like that specific example, too. I think it would put – it put my mind at ease. I think it would put other people’s mind at ease about the protection of personal information. |
| Researcher availability | You may contact [study PI] if you have any questions or concerns. I appreciate that statement right there, because sometimes I do have questions. I would like to talk directly to someone instead of being told to call back or talk to a machine. |
| Researcher and/or institutional expertise | It’s good to know that you have to have special training to actually authorize and access people’s records. |
| Not sharing personal information with drug companies | To be seen by or sold to drug companies. Thank God. Oh, goodness. Okay, I chuckle at that, because particularly seniors already pay through the nose about drug companies. I wouldn’t trust their ethics or their motivation for getting the information. |
| P: That’s cool. That’s interesting. My health record cannot be sold by or seen by the drug companies. | |
| I: Why is that interesting to you? | |
| P: I wouldn’t want phone calls from different drug companies, so I would think that would be a good thing. Getting phone calls, “Try this drug. Try this drug.” | |
| Rights as research subject | P: “No studies on people can be conducted at the University of Florida without a thorough review of the ethics of the study and rigorous protection of the patient.” Okay. In other words, they have to come and ask me. They can’t do a study on me unless they come to me and get my consent. Correct? |
| I: That’s what you understand it. | |
| P: Okay. That is something that is noted that is a positive. | |
| Then I like the no cost for the participation. |
DISCUSSION
In the context of consenting to share health record data for research, this study provides preliminary support for the value of electronic applications with interactive features that allow patients to customize their consent experience. At the same time, this study suggests a need to support people who have reservations about electronic consent platforms as well as the importance of communicating information about administrative processes and safeguards that protect personal health information when used in research.
In this study, some participants expressed interest in clicking further to read more information than is required by consent regulations. However, other participants focused on the primary information and did not explore more detailed information. This behavior, if it generalizes to broader patient populations, would highlight an advantage of electronic consent applications that interactively accommodate high-information-seeking users without overwhelming low-information-seeking users with more details than they desire.
Relatedly, the study aligns with previous research indicating that patients seek and value information about data protections and the specific purpose of the study to which they are consenting.17,27 For many participants in this study, it was insufficient to simply indicate that their EHR data would be kept secure. These participants desired specifics about what information is contained in their EHR, where their data are stored, which safeguards are applied, who has access to their data, and how breaches or misuses will be addressed. Moreover, some participants raised questions about the duration of their consent and how different researchers would gain access to these data. Notably, the updated Common Rule requires that broad consent must include descriptions of the types and purposes of research that could be conducted; the types of identifiable information that might be used, including whether sharing might occur and the types of institutions or researchers that might access these data; and the time period over which data could be used. Overall, these findings point to an ongoing challenge for broad consent implementation, namely the ability to carefully balance the delivery of information that is specific enough to satisfy patients’ information needs but also general enough to allow broad future research uses. Again, electronic platforms may help strike this balance through interactive capabilities and other functionalities.
Previous work has also identified the importance of trust in researchers when it comes to people’s willingness to participate in research.13,46,47 Trust in the source of information is critical to personal evaluations of risk information.48–50 Several topics and statements in this study’s e-consent application were described as reassuring or “good to know,” including details about data protections, required researcher training, and the rights of study participants. Therefore, this finding is consistent with prior work indicating the importance of transparency and with efforts to present consent information in the context of a trusted relationship. However, it is important to note that our sample was largely composed of older, female, and white participants. Therefore, the nature and extent of reassurances expressed in these interviews may not generalize to other types of patients – particularly those from minority populations, who have consistently expressed lower levels of trust in medical researchers.51–53 Future research should involve larger and more diverse participant samples to fully understand people’s trust and use of e-consent for deciding whether or not to share their health records for research.
Additionally, findings from this study align with previous research indicating that patients have more concerns about giving their data to pharmaceutical companies than to academic institutions and hospitals.16,21,24,25,27 A majority of patients in this study expressed misgivings about pharmaceutical companies having access to their data, for reasons ranging from privacy concerns to questions about the companies’ motivations for obtaining data. Additionally, when prompted by a statement in the e-consent application informing patients that information in their medical record would not be divulged to the federal government except in “special circumstances,” participants also raised concerns about government access to their data. Therefore, broad consent applications might need to clearly describe when and why industry and government entities would receive research data.
With regard to using an electronic application, patients expressed divergent attitudes and preferences. Many participants favored the idea of consenting electronically rather than on paper, citing the streamlined organization and clarity of the information provided on a tablet. Other strengths of an electronic consent format as described by participants include the associated reduction in paperwork and the interactive capabilities of the application. Some participants referenced familiarity with the use of tablet computers in other settings, such as other health care settings and restaurants.
However, several participants expressed either a preference for completing a consent form on paper or concerns about using a tablet. Also, while our qualitative data do not allow for age-based comparisons, many participants expressed concerns about the ability of older adults to consent on an electronic device, either speaking for themselves or drawing on the experiences of family members or friends. Although national trends indicate that 68% of adults in the United States own a touchscreen smartphone and that nearly half own tablet computers, these numbers shrink significantly among Americans age 65 and older, with only one-third reporting ownership of a smartphone or tablet computer.54 Future efforts to introduce consent in an electronic format should consider the target patient population and the costs and benefits of offering paper or non–tablet computer alternatives to accommodate some patients.
This study has several strengths, including the detailed think-aloud interview protocol and the iterative multicoder process used to analyze the data. However, there are also several limitations. First, we studied a moderate-size sample of people in north-central Florida. Thus, our findings may not represent the diversity of attitudes and preferences held by a broader population. Related to this, while we aimed to capture a range of perspectives from patients who differed demographically, male, younger, and Hispanic patients were underrepresented relative to other groups. Finally, this study examined technology use and information-seeking behavior in a laboratory environment. Because of this, results may not generalize to typical environments in which consenting occurs, such as medical clinics and hospitals.
Given this study’s findings and its limitations, there is a need for future research, including larger experimental studies that examine the relative effectiveness when different interactive electronic capabilities and content are used in broad consent for research use of EHR data. To that end, we used the findings from this study to update the content of our e-consent application, including adding clearer and more detailed information on when and how researchers or other organizations might use EHR data. This updated application will be used in a future randomized trial that tests the effects of varying levels of information and interactivity on patients’ decisions to share their EHR data for research. Other future research could include direct comparisons of e-consent to paper-based consent to better understand whether and how the electronic medium affects the consent process and outcomes.
CONCLUSIONS
This study contributes new insights on how e-consent applications could be designed to ensure that patients’ information needs are met when seeking consent for research use of health record information. Also, this study offers a potential electronic approach to meeting the new Common Rule requirement that consent documents contain a “concise and focused” presentation of key information followed by more details.
COMPETING INTERESTS
The authors have no competing interests to declare.
FUNDING
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award no. R01HD086700. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONTRIBUTORS
CAH was responsible for the study concept and design, overseeing data collection, analysis and interpretation of the data, and initial drafting of the manuscript. EHG was responsible for analysis and interpretation of the data and initial drafting of the manuscript. KPR was responsible for design, collection, analysis, and interpretation of the data. DH was responsible for design, collection, and interpretation of the data. JLK, AGM, and REM were responsible for the study concept and design and overseeing data collection. All authors contributed to revising the manuscript, approved the data interpretation, and approved the final manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Dr Ken Goodman and Peter Iafrate for their feedback and guidance on the e-consent application content and development. The authors also acknowledge the University of Florida HealthStreet staff for support in participant recruitment.
REFERENCES
- 1. Lowes LP, Noritz GH, Newmeyer A, Embi PJ, Yin H, Smoyer WE. ‘Learn From Every Patient’: Implementation and early results of a learning health system. Dev Med Child Neurol. 2017;592:183–91. [DOI] [PubMed] [Google Scholar]
- 2. Embi PJ, Payne PR. Evidence generating medicine: redefining the research-practice relationship to complete the evidence cycle. Med Care. 2013;51:S87–91. [DOI] [PubMed] [Google Scholar]
- 3. McGlynn EA, Lieu TA, Durham ML, et al. Developing a data infrastructure for a learning health system: the PORTAL network. J Am Med Inform Assoc. 2014;214:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hernandez AF, Fleurence RL, Rothman RL. The ADAPTABLE trial and PCORnet: shining light on a new research paradigm. Ann Int Med. 2015;1638:635–36. [DOI] [PubMed] [Google Scholar]
- 5. Ohno-Machado L, Alipanah N, Day M, et al. Comprehensive Inventory of Research Networks: Clinical Data Research Networks, Patient-Powered Research Networks, and Patient Registries. Washington, DC: Patient Centered Outcomes Research Institute, 2013. [Google Scholar]
- 6. Clayton EW, Smith M, Fullerton SM, et al. Confronting real time ethical, legal, and social issues in the Electronic Medical Records and Genomics (eMERGE) Consortium. Genet Med. 2010;1210:616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kohane IS. Using electronic health records to drive discovery in disease genomics. Nat Rev Genet. 2011;126:417–28. [DOI] [PubMed] [Google Scholar]
- 8. Bowton E, Field JR, Wang S, et al. Biobanks and electronic medical records: enabling cost-effective research. Sci Transl Med. 2014;6234:234cm3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sitapati A, Kim H, Berkovich B, et al. Integrated precision medicine: the role of electronic health records in delivering personalized treatment. Wiley Interdiscip Rev Sys Biol Med. 2017;93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thadani SR, Weng C, Bigger JT, Ennever JF, Wajngurt D. Electronic screening improves efficiency in clinical trial recruitment. J Am Med Inform Assoc. 2009;166:869–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byrne MM, Tannenbaum SL, Glück S, Hurley J, Antoni M. Participation in cancer clinical trials: why are patients not participating? Med Decis Making. 2014;341:116–26. [DOI] [PubMed] [Google Scholar]
- 12. Corbie-Smith G, Thomas S, Williams M, Moody-Ayers S. Attitudes and beliefs of African-Americans toward participation in medical research. General Int Med. 1999;149:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corbie-Smith G, Thomas SB St. George DMM. Distrust, race, and research. Arch Int Med. 2002;16221:2458–63. [DOI] [PubMed] [Google Scholar]
- 14. Larson E. Exclusion of certain groups from clinical research. J Nurs Scholarship. 1994;263:185–90. [DOI] [PubMed] [Google Scholar]
- 15. Stone VE, Mauch MY, Steger K, Janas SF, Craven DE. Race, gender, drug use, and participation in AIDS clinical trials. General Int Med. 1997;123:150–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weitzman ER, Kaci L, Mandl KD. Sharing medical data for health research: the early personal health record experience. J Med Internet Res. 2010;122:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim KK, Joseph JG, Ohno-Machado L. Comparison of consumers’ views on electronic data sharing for healthcare and research. J Am Med Inform Assoc 2015;224:821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kass NE, Natowicz MR, Hull SC, et al. The use of medical records in research: what do patients want? J Law, Med Ethics. 2003;313:429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Riordan F, Papoutsi C, Reed JE, Marston C, Bell D, Majeed A. Patient and public attitudes towards informed consent models and levels of awareness of electronic health records in the UK. Int J Med Inform. 2015;844:237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nair K, Willison D, Holbrook A, Keshavjee K. Patients’ consent preferences regarding the use of their health information for research purposes: a qualitative study. J Health Serv Res Policy. 2004;91:22–27. [DOI] [PubMed] [Google Scholar]
- 21. Willison DJ, Schwartz L, Abelson J, et al. Alternatives to project-specific consent for access to personal information for health research: what is the opinion of the Canadian public? J Am Med Inform Assoc. 2007;146:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mamo LA, Browe DK, Logan HC, Kim KK. Patient-informed governance of distributed research networks: Results and discussion from six patient focus groups. AMIA Annu Symp Proc. 2013;2013:920–29. [PMC free article] [PubMed] [Google Scholar]
- 23. Willison DJ, Keshavjee K, Nair K, Goldsmith C, Holbrook AM. Patients’ consent preferences for research uses of information in electronic medical records: interview and survey data. BMJ. 2003;3267385:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Willison DJ, Steeves V, Charles C, et al. Consent for use of personal information for health research: do people with potentially stigmatizing health conditions and the general public differ in their opinions? BMC Med Ethics. 2009;101:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willison DJ, Swinton M, Schwartz L, et al. Alternatives to project-specific consent for access to personal information for health research: insights from a public dialogue. BMC Med Ethics. 2008;91:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simon CM, L’heureux J, Murray JC, et al. Active choice but not too active: public perspectives on biobank consent models. Genet Med. 2011;139:821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grande D, Mitra N, Shah A, Wan F, Asch DA. Public preferences about secondary uses of electronic health information. JAMA Int Med. 2013;17319:1798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morillo F, Bordons M, Gómez I. Interdisciplinarity in science: a tentative typology of disciplines and research areas. J Am Soc Inform Sci Technol. 2003;5413:1237–49. [Google Scholar]
- 29. Wendler D. One-time general consent for research on biological samples: is it compatible with the Health Insurance Portability and Accountability Act? Arch Int Med. 2006;16614:1449–52. [DOI] [PubMed] [Google Scholar]
- 30. Jimison HB, Sher PP, Appleyard R, LeVernois Y. The use of multimedia in the informed consent process. J Am Med Inform Assoc. 1998;53:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ryan R, Prictor M, McLaughlin KJ, Hill S. Audio-visual presentation of information for informed consent for participation in clinical trials. Cochrane Database Syst Rev. 2008;231:CD003717. [DOI] [PubMed] [Google Scholar]
- 32. Sonne SC, Andrews JO, Gentilin SM, et al. Development and pilot testing of a video-assisted informed consent process. Contemp Clin Trials. 2013;361:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Armstrong AW, Alikhan A, Cheng L, Schupp C, Kurlinkus C, Eisen DB. Portable video media for presenting informed consent and wound care instructions for skin biopsies: a randomized controlled trial. Brit J Dermatol. 2010;1635:1014–19. [DOI] [PubMed] [Google Scholar]
- 34. Kass NE, Sugarman J, Medley AM, et al. An intervention to improve cancer patients’ understanding of early-phase clinical trials. IRB: Ethics Human Res. 2009;313:1–10. [PMC free article] [PubMed] [Google Scholar]
- 35. Marshall EA, Oates JC, Shoaibi A, et al. A population-based approach for implementing change from opt-out to opt-in research permissions. PLoS One. 2017;124:e0168223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iafrate P, Lipori GP, Harle CA, et al. Consent2Share: an integrated broad consenting process for re-contacting potential study subjects. J Clin Transl Res. 2016;24:113–22. [PMC free article] [PubMed] [Google Scholar]
- 37. Shneiderman B. The eyes have it: a task by data type taxonomy for information visualizations. Proceedings of the 1996 IEEE Symposium on Visual Languages. 1996:336–43. [Google Scholar]
- 38. Ericsson KA, Simon HA. Verbal reports as data. Psychol Rev. 1980;873:215–51. [Google Scholar]
- 39. Ericsson KA, Simon HA. Protocol Analysis: Verbal Reports as Data. Cambridge, MA: Bradford Books/MIT Press; 1984. [Google Scholar]
- 40. Harle CA, Padman R, Downs JS. Designing a personalized health risk communication website to motivate user attention and systematic processing. Proceedings of the Seventh Annual Pre-ICIS Workshop on HCI Research in MIS. Paris: Association for Information Systems; 2009. [Google Scholar]
- 41. Palgliari C. Design and evaluation in eHealth: Challenges and implications for an interdisciplinary field. J Med Internet Res. 2007;92:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Corbin JM, Strauss A. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;131:3–21. [Google Scholar]
- 43. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;201:37–46. [Google Scholar]
- 44. Miles MB, Huberman AM. Qualitative Data Analysis: A Sourcebook. Beverly Hills, CA: Sage Publications; 1994. [Google Scholar]
- 45. McHugh ML. Interrater reliability: the kappa statistic. Biochemia Med. 2012;223:276–82. [PMC free article] [PubMed] [Google Scholar]
- 46. Mainous AG, Smith DW, Geesey ME, Tilley BC. Development of a measure to assess patient trust in medical researchers. Ann Fam Med. 2006;43:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;124:248–56. [DOI] [PubMed] [Google Scholar]
- 48. Fischhoff B, Lichtenstein S, Slovic P, Derby S, Keeney R. Acceptable Risk. New York: Cambridge University Press; 1981. [Google Scholar]
- 49. Peters RG, Covello VT, McCallum DB. The determinants of trust and credibility in environmental risk communication: an empirical study. Risk Analysis. 1997;171:43–54. [DOI] [PubMed] [Google Scholar]
- 50. Reynolds BJ. When the facts are just not enough: credibly communicating about risk is riskier when emotions run high and time is short. Toxicol Appl Pharmacol. 2011;2542:206–14. [DOI] [PubMed] [Google Scholar]
- 51. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine. 2008;871:1–9. [DOI] [PubMed] [Google Scholar]
- 52. Bussey-Jones J, Garrett J, Henderson G, Moloney M, Blumenthal C, Corbie-Smith G. The role of race and trust in tissue/blood donation for genetic research. Genet Med. 2010;122:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bakken S, Lantigua RA, Busacca LV, Bigger JT. Barriers, enablers, and incentives for research participation: a report from the Ambulatory Care Research Network (ACRN). J Am Board Fam Med. 2009;224:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anderson M. The Demographics of Device Ownership. Washington, DC: Pew Research Center; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


