Fig. 1. Acetylation of human NEIL1 by p300 and identification of acetyl-accepting residues.
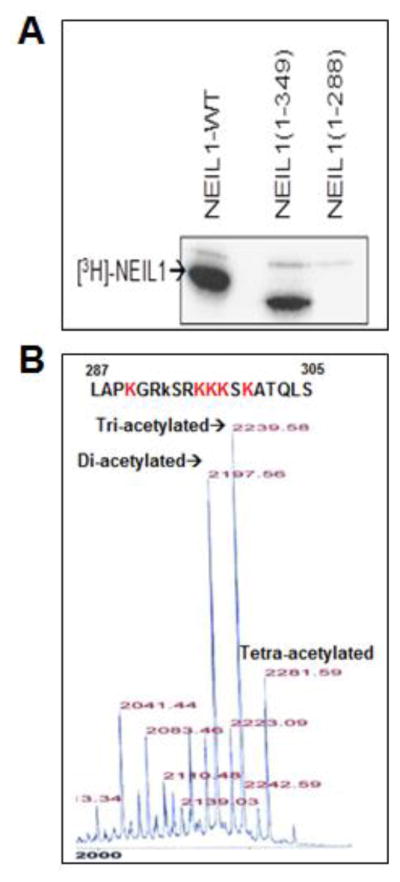
(A) Recombinant full-length WT, truncated N1-349 or N1-288 NEIL1 were acetylated in vitro with p300 HAT and 1 μCi of [3H] AcCoA (200 mCi/mM) in HAT buffer (details in Materials and Methods), followed by SDS-PAGE and fluorography.
(B) Mass spectrometric analysis of a synthetic NEIL1 peptide (aa 287-305) after in vitro acetylation with p300 HAT and AcCoA, which identified tri-acetylated NEIL1 as the predominant acetylated form with K296, K297 and K298 as the major acetyl-accepting residues.
