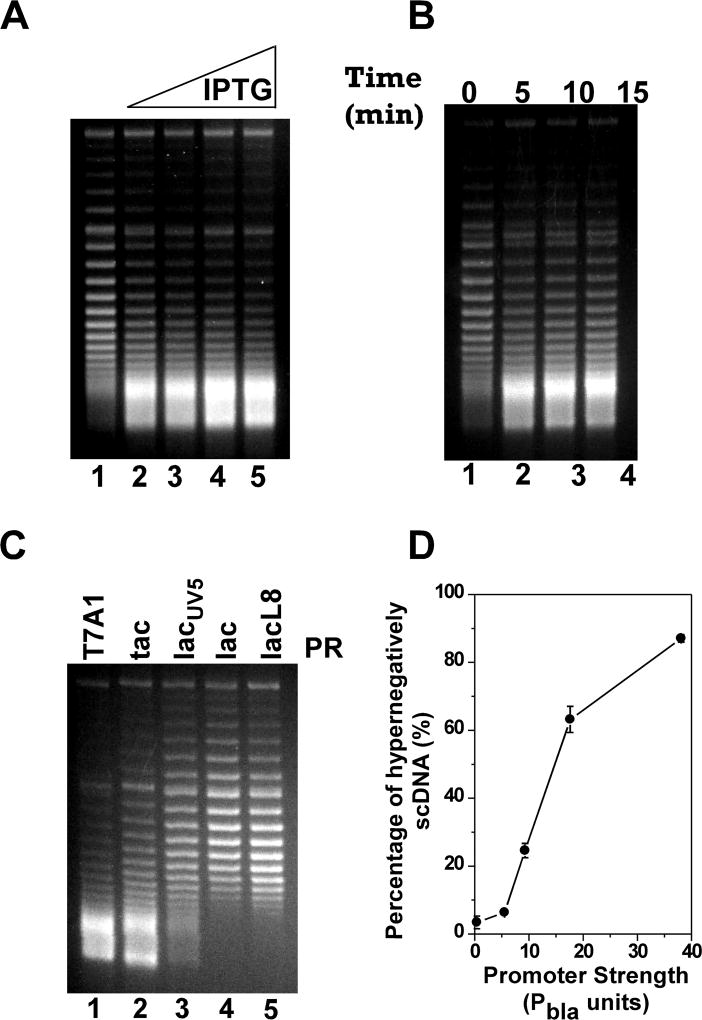
Figure 3. TCDS in E. coli strain VS111 is dependent on promoter strength.
The in vivo T-S assays were performed as described under Experimental Procedures. DNA topoisomers were resolved by electrophoresis in a 1% agarose gel containing 2.5 µg/ml chloroquine and stained with ethidium bromide. The fastest moving band in the gels where DNA topoisomers are no longer resolvable under our experimental conditions represents the hypernegatively supercoiled DNA. (A) Dependence of TCDS on IPTG concentration for plasmid pZXD44 carrying a tet gene under the control of the strong PT7A1/O4 promoter. Lane 1 contained the DNA sample isolated from E. coli cells prior to IPTG induction. Lanes 2–5 contained the DNA samples isolated from E. coli cells after 10 min of induction with 25, 50, 100, and 1000 µM IPTG, respectively. (B) Time course of the hypernegative supercoiling of plasmid pZXD44 in E. coli strain VS111 after 1 mM of IPTG induction. Lanes 1–4 contained, respectively, DNA samples isolated from VS111 after 0, 5, 10, and 15 min of IPTG induction. (C) Dependence of TCDS on promoter strength. Lanes 1–5 contained, respectively, DNA samples isolated from E. coli topA strain VS111 containing plasmids pZXD44, 50, 49, 47, and 48 after 5 min of IPTG (1 mM) induction. These plasmids carry a tet gene under the control of IPTG-inducible promoters with different strengths, i.e., promoters PT7A1/O4, Ptac, PlacUV5, Plac, and PlacL8. Labels: PR, promoter; T7A1, the T7A1/O4 promoter; tac, the tac promoter; lacUV5, the lacUV5 promoter; lac, the lac promoter; lacL8, the lacL8 promoter. (D) The percentage of hypernegatively supercoiled DNA is proportional to promoter strength (the values are the average of at least three independent determinations and the standard deviations are shown). These results were calculated as described under Experimental Procedures using the TCDS data shown in (C). Promoter strength in Pbla units was obtained from ref. (Lanzer and Bujard 1988)