Abstract
Military personnel experience posttraumatic stress disorder (PTSD), which is associated with differential DNA methylation across the whole genome. However, the relationship between these DNA methylation patterns and clinically relevant increases in PTSD severity is not yet clearly understood. The purpose of this study was to identify differences in DNA methylation associated with PTSD symptoms and investigate DNA methylation changes related to increases in the severity of PTSD in military personnel. In this pilot study, a cross-sectional comparison was made between military personnel with PTSD (n = 8) and combat-matched controls without PTSD (n = 6). Symptom measures were obtained, and genome-wide DNA methylation was measured using methylated DNA immunoprecipitation (MeDIP-seq) from whole blood samples at baseline and 3 months later. A longitudinal comparison measured DNA methylation changes in military personnel with clinically relevant increases in PTSD symptoms between time points (PTSD onset) and compared methylation patterns to controls with no clinical changes in PTSD. In military personnel with elevated PTSD symptoms 3 months following baseline, 119 genes exhibited reduced methylation and 8 genes exhibited increased methylation. Genes with reduced methylation in the PTSD-onset group relate to the canonical pathways of netrin signaling, Wnt/Ca+ pathway, and axonal guidance signaling. These gene pathways relate to neurological disorders, and the current findings suggest that these epigenetic changes potentially relate to PTSD symptomology. This study provides some novel insights into the role of epigenetic changes in PTSD symptoms and the progression of PTSD symptoms in military personnel.
Keywords: PTSD, DNA methylation, biomarkers, military
Posttraumatic stress disorder (PTSD) is a trauma/stress-related disorder that is initiated by psychologically distressing events such as combat or physical assault (Hughes & Shin, 2011; Xue et al., 2015). Among the more than 2 million U.S. military personnel who deployed to Iraq and Afghanistan, approximately 153,000 were diagnosed with PTSD after returning from military duty (Fischer, 2014; Fisher, 2014). Costs of treatment over 2 years for combat-related PTSD are estimated to be around US$2 billion (Cesur, Sabia, & Tekin, 2013). PTSD symptoms, including intrusion, avoidance, negative alterations in cognition and mood, and alterations in arousal and reactivity, can last for months or even years after an individual’s return from deployment (American Psychiatric Assocaition 2013). PTSD is therefore associated with dramatic reductions in health-related quality of life that have longlasting impacts on all area of health and well-being (Bush, 2014).
Previous studies have investigated the role of genes in PTSD symptoms, demonstrating associations between these symptoms and genes related to serotonergic, dopaminergic systems, and the hypothalamic–pituitary–adrenal axis (Almli, Fani, Smith, & Ressler, 2014; Rampp, Binder, & Provencal, 2014). The expression of these genes is modulated by many epigenetic mechanisms. DNA methylation, a major epigenetic mechanism for regulating gene expression, occurs by the covalent transfer of a methyl group to the five position of cytosine residues of DNA by DNA methyltransferases, which results in differential gene activity (Robertson, 2005). Canonically, increased DNA methylation (hypermethylation) in the promoter region of a gene reduces gene expression, whereas reduced DNA methylation (hypomethylation) increases gene expression.
Various lifetime experiences may alter DNA methylation profiles, which then affect downstream expression of genes (Rampp et al., 2014). Traumatic events from deployment or war could cause DNA methylation changes, which could then affect PTSD symptoms (Almli et al., 2014). In military personnel, increased methylation of NR3C1-1F promoter was related to more severe symptoms of PTSD (Yehuda et al., 2015), and decreased methylation the of FKBP5 gene exon 1 (FKBP51) promoter region was associated with recovery from PTSD after psychotherapy (Yehuda et al., 2013). Methylation of SKA2 was associated with more severe PTSD symptoms and with cortical stress reactivity in veterans (Boks et al., 2016; Sadeh et al., 2016).
Although DNA methylation is associated with PTSD in military personnel and veterans, much less is known regarding the role of methylation in the onset of PTSD or increases in symptom severity at the whole genome level. To address this, in the present pilot study, we examined a cohort of military personnel returning from deployment and hypothesized that changes in DNA methylation would relate to clinically relevant increases in PTSD symptoms over a 3-month period.
Materials and Method
Participants
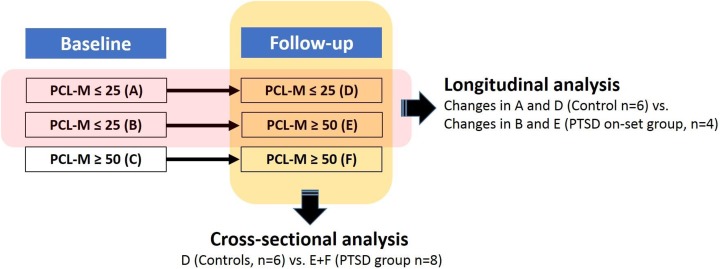
This pilot study was a case–control comparison between 2 time points based on PTSD symptom change among active-duty personnel (Figure 1 illustrates the study design). The institutional review board approved this study prior to data collection, and we obtained written informed consent from all participants. This sample was part of a larger military cohort recruited from a sleep clinic at Madigan Army Medical Center (Mysliwiec et al., 2013). Participants were active-duty military personnel who had returned from deployment to Afghanistan (Operation Enduring Freedom) or Iraq (Operation Iraqi Freedom) within the prior 18 months and were seeking care for sleep disturbances. Exclusion criteria included (1) history of drug or alcohol abuse in the previous year, (2) current severe medical conditions requiring chronic treatment (e.g., cancer, diabetes, HIV, and autoimmune disorders) or a severe psychiatric condition (i.e., schizophrenia, bipolar disorder), and (3) severe neurological disorders (e.g., multiple sclerosis, seizure disorders, and history of stroke). Because PTSD in military populations often co-occurs with combat injuries like traumatic brain injury (TBI) and comorbid psychological disorders such as depression, we did not exclude subjects with these conditions.
Figure 1.
Study design. Participants were grouped by score on the Post-traumatic Stress Disorder Checklist–Military Version (PCL-M) at both time points assessed. In the longitudinal analysis, changes to methylation patterns in the posttraumatic stress disorder (PTSD)-onset group between time points (B and E) were analyzed and then compared with changes in the control group between time points (A and D). In the cross-sectional analysis, methylation patterns of all participants who met PTSD criteria at the follow-up time point (E and F) were compared to controls at follow-up (D).
For the longitudinal analysis, we compared changes in DNA methylation from baseline to 3-month follow-up between participants whose symptoms did not meet the criteria for PTSD at baseline but had worsened to meet those criteria by follow-up (PTSD onset) and participants whose symptoms did not meet the criteria for PTSD at either time point (no-PTSD controls). For the cross-sectional analysis, we compared methylation patterns at follow-up between all participants whose symptoms met the criteria for PTSD at follow-up (PTSD) and whose symptoms did not meet these criteria at follow-up (no-PTSD controls).
Clinical Symptoms Measurement
We measured PTSD symptoms using the PTSD Checklist Military Version (PCL-M). Possible score ranges from 17 (lowest severity) to 85 (highest severity), with a score of 50 or more indicating a clinical diagnosis of PTSD as determined by a structured clinical interview. In the present study, we conformed to the military standard that a score of 50 or higher is a diagnostic indicator of PTSD and grouped participants based on this 50-point cutoff. We measured depression symptoms using the Quick Inventory of Depressive Symptomatology (QIDS; Rush et al., 2003). We administered the PCL-M and QIDS at both baseline and follow-up. Participants identified TBI via self-reporting on the Warrior Administered Retrospective Casualty Assessment Tool (Terrio, Nelson, Betthauser, Harwood, & Brenner, 2011).
Genome-wide DNA Methylation Profiling Using MeDIP-seq
We collected peripheral blood at baseline and follow-up from nonfasting participants. Genomic DNA was extracted from whole blood using QIAamp DNA Blood Maxi Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. To obtain the methylated DNA, 2.5 μg of isolated genomic DNA was fragmented down to 250 bp using an M220 Focused-ultrasonicator (Covaris, Woburn, MA), then 1 μg fragmented DNA was processed with MethylMiner Methylated DNA Enrichment Kit (Invitrogen, Carlsbad, CA), which resulted in selection, via immunoprecipitation, of DNA fragments containing methylated CpG. Enriched methylated-DNA fragments (10 ng) were used for a DNA library preparation with the TruSeq Nano DNA kit (Illumina, San Diego, CA). The HiSeq 2500 system was used to sequence with a paired-end method, where both ends of a DNA fragment are sequenced, with 101 base pairs read from each end of the fragment with TruSeq Rapid SBS kit (Illumina) for the sequencing of the DNA library. Sequencing resolution allows identification of regions that are methylated, which were mapped to the gene level during the analysis. Macrogen Corp. (Rockville, MD) performed DNA library preparation and sequencing.
Statistical Analysis
To compare demographic and clinical characteristics between participants with PTSD and the no-PTSD controls and between participants with PTSD onset during the study and the no-PTSD controls, we employed two-tailed χ2 tests and t tests using SPSS Version 22.0 (SPSS Inc., Armonk, New York). We compared symptom inventory scores between groups using independent samples t tests and TBI using χ2 test. We considered differences to be statistically significant when p < .05.
We assessed base call quality on FASTQ files, which is a raw data format that contains unaligned reads with a quality score for each base call, using FASTQC Version 0.10.1. Reads were aligned to the reference human genome of hg19 from UCSC using a short read aligner, BWA Version 0.7.8. Peaks were called using MACS Version 2.1.0.20140616 to identify DNA regions that were methylated. For identification of differentially methylated DNA regions between the participants with PTSD and the control group in the cross-sectional analysis as well as baseline and follow-up in the longitudinal analysis, we used the DiffBind (Version 2.6.6) software package. The binding affinity matrix was generated by counting sequenced reads overlapping intervals in peak sets. A differential binding affinity analysis was employed to identify statistically significant differences in methylated peak intervals between groups with a false discovery rate (FDR) = 0.10. We used a higher FDR cutoff than the conventionally used value of 0.05 to reduce the false-negative rate considering this pilot study’s small sample size. To identify molecular pathways related to the differentially methylated genes as well as top-gene networks implicated in these gene-regulation changes associated with PTSD, we used QIAGEN’s Ingenuity Pathway Analysis (IPA, QIAGEN, Venlo, Limburg, the Netherlands). (Details of these analyses are provided in the Online Supplementary Appendix.)
Results
Demographic and Clinical Characteristics
Longitudinal analysis
Our longitudinal analysis included PTSD-onset participants (n = 4), whose PCL-M scores increased between the baseline and follow-up assessments to above the 50-point threshold (mean = 46.25, standard deviation [SD] = 2.9 at baseline; mean = 59.25, SD = 3.5 at follow-up), with an increase of >10 points (Δmean = 13, ΔSD = 1.63) in each participant and no-PTSD control participants (n = 6). Participants in this analysis were an average of 39.0 years old (SD = 7.6), were male, and were 50% Caucasian. No-PTSD control subjects had PCL-M scores ≤25 at both time points (mean = 23.33, SD = 1.6; mean = 22.67, SD = 1.6, respectively). There were no significant differences between groups in demographic characteristics including age, body mass index, race, education, medication use, and the number of deployments. Significantly more participants reported a previous TBI in the PTSD-onset group (100% in PTSD-onset group and 16.7% in controls, p = .003). PCL-M and QIDS scores were significantly higher in the PTSD-onset group as compared to the no-PTSD controls at both time points.
Cross-sectional analysis
At the time point assessed for this analysis (3-month follow-up), PTSD participants (n = 8) had a PCL-M score ≥50 (mean = 60.38, SD = 5.5), while no-PTSD controls (n = 6) had a PCL-M score less than or equal to 25 (mean = 22.67, SD = 1.6). Demographic and clinical characteristics of the participants included in the cross-sectional analysis (n = 14) are shown in Table 1. There were no significant differences in the demographic characteristics between the two groups. However, the PTSD group had significantly higher scores on both the PCL-M and the QIDS at baseline and follow-up.
Table 1.
Demographic and Clinical Characteristics for the PTSD and no-PTSD Control Groups.
| Characteristic | Total (n = 14) | PTSDa (n = 8) | No-PTSDb (n = 6) | x 2/t | p Value |
|---|---|---|---|---|---|
| Age (years), M (SD) | 35.07 (8.9) | 32.25 (8.9) | 38.83 (8.1) | 1.423 | .180 |
| Sex (males), n (%) | 14 (100.0) | 8 (100.0) | 6 (100.0) | ||
| Race (White) n (%) | 6 (46.2) | 4 (50.0) | 2 (33.3) | 0.737 | .592 |
| Education (years), M (SD) | 14.14 (2.1) | 14.38 (3.2) | 13.83 (1.0) | −0.453 | .658 |
| BMI (kg/m2), M (SD) | 30.63 (3.7) | 30.20 (4.2) | 31.23 (3.2) | 0.520 | .613 |
| Medication use,c n (%) | 8 (57.1) | 5 (71.4) | 3 (50) | 0.627 | .592 |
| Number of deployments, M (SD) | 2.21 (1.2) | 1.75 (1.0) | 2.83 (1.2) | 1.835 | .091 |
| mTBI diagnosis, n (%) | 9 (64.3) | 8 (100.0) | 1 (16.7) | 10.370 | .003 |
| PCL-M, M (SD) | |||||
| Baseline | 40.36 (16.6) | 53.13 (8.7) | 23.33 (1.6) | <.001 | |
| Follow-up | 44.21 (19.8) | 60.38 (5.5) | 22.67 (1.6) | −16.117 | <.001 |
| QIDS, M (SD) | |||||
| Baseline | 11.14 (5.5) | 15.13 (3.0) | 5.83 (2.6) | <.001 | |
| Follow-up | 11.43 (5.3) | 15.38 (2.3) | 6.17 (2.6) | −7.024 | <.001 |
Note. BMI = body mass index; mTBI = mild traumatic brain injury; QIDS = Quick Inventory of Depressive Symptomatology; PCL-M = PTSD Checklist–Military Version; PTSD = posttraumatic stress disorder.
aPTSD group had PCL-M scores ≥50 at follow-up. bNo-PTSD control group had PCL-M scores ≤25 at follow-up. cMedications included antidepressants, antipsychotics, benzodiazepines, nonbenzodiazepines, narcotics, and prazosin.
Differentially Methylated Genes
Longitudinal analysis
We observed significant alterations in methylation from baseline to the 3-month follow-up in participants in the PTSD-onset group, while in the control group only one nonoverlapping region showed statistically significant DNA methylation changes between time points. There were 124 regions, which corresponded to 119 genes, that significantly decreased in methylation from baseline to follow-up in the PTSD-onset group (see Table 2 for top 10 genes, full gene list in Online Supplemental Table 1). The top canonical pathways, according to IPA analysis, for the genes that decreased in methylation included netrin signaling, Wnt/Ca+ pathway, and axonal guidance signaling (Table 3). Conversely, there were eight genes that significantly increased in methylation between baseline and follow-up in the PTSD-onset group (Table 4). As mentioned above, one unique gene interval in the intronic region of FAM178B had significantly increased methylation between baseline and follow-up in the controls only.
Table 2.
Top Genes With Decreased Methylation From Baseline to Follow Up in the PTSD-Onset Group.
| Gene Symbol | Description | Adjusted p Value (FDR) | Fold Change |
|---|---|---|---|
| ROCK1P1 | Rho-associated, coiled-coil containing protein kinase 1 pseudogene 1 | .02223 | 6.41446 |
| EPPK1 | Epiplakin 1, mRNA | .02632 | 6.09928 |
| NFATC1 | Nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1, transcript variant 2 | .04530 | 5.73130 |
| USP41 | Ubiquitin-specific peptidase 41 | .04053 | 5.55615 |
| JAKMIP3 | Janus kinase and microtubule interacting protein 3 | .03204 | 5.46414 |
| INTS1 | Integrator complex subunit 1 | .03204 | 5.38736 |
| SLC12A7 | Solute carrier family 12 (potassium/chloride transporters), member 7 | .04528 | 5.34616 |
| COL6A1 | Collagen, type VI, α1 | .04530 | 5.30660 |
| KIAA1530 | KIAA1530 | .02223 | 5.30433 |
| CSNK1G2 | Casein kinase 1, γ 2 | .05454 | 5.30192 |
Note. PTSD-onset group had scores on the PTSD Checklist–Military Version (PCL-M) <50 at baseline (i.e., no diagnosis of PTSD) and ≥50 at follow-up (i.e., diagnosis of PTSD). FDR = false discovery rate; PTSD = posttraumatic stress disorder.
Table 3.
Ingenuity Pathway Analysis (IPA) Canonical Pathways of Differentially Methylated Genes in the PTSD-onset Group.
| Pathway | p Value | Overlap |
|---|---|---|
| Netrin signaling | .00111 | 7.7% (3/39) |
| G protein signaling | .00259 | 3.7% (4/109) |
| Wnt/Ca+ pathway | .00315 | 5.4% (3/56) |
| Huntington’s disease signaling | .00711 | 2.2% (5/229) |
| Axonal guidance signaling | .00783 | 1.6% (7/434) |
Note. Posttraumatic stress disorder (PTSD)-onset group had scores on the PTSD Checklist–Military Version (PCL-M) <50 at baseline (i.e., no diagnosis of PTSD) and ≥50 at follow-up (i.e., diagnosis of PTSD).
Table 4.
Genes With Increased Methylation From Baseline to Follow Up in the PTSD-Onset Group.
| Gene Symbol | Description | Adjusted p Value (FDR) | Fold Change |
|---|---|---|---|
| ANKRD30BL | Ankyrin repeat domain 30B-like | .08837 | 5.5588 |
| AK125749 | cDNA FLJ43761 fis, clone TESTI2048109 | .03204 | 4.3178 |
| GRK4 | G protein-coupled receptor kinase 4 | .04710 | 3.9876 |
| LRPAP1 | LDL receptor related protein associated protein 1 | .04710 | 3.9381 |
| NCS1 | Neuronal calcium sensor 1 | .04530 | 3.8335 |
| PCYOX1 | Prenylcysteine oxidase 1 | .07389 | 3.6540 |
| ZFHX3 | Zinc finger homeobox 3 | .06857 | 3.7169 |
| GJC1 | Gap junction protein gamma 1 | .07342 | 3.3321 |
Note. PTSD-onset group had scores on the PTSD Checklist–Military Version (PCL-M) <50 at baseline (i.e., no diagnosis of PTSD) and ≥50 at follow-up (i.e., diagnosis of PTSD). FDR = false discovery rate; PTSD = posttraumatic stress disorder.
Cross-sectional analysis
In all PTSD subjects at follow-up, 10 genes were significantly hypermethylated compared to controls at follow-up, including METTL16, TMEM242, and PNPLA7, and 18 genes were significantly hypomethylated, including MYO1D, FLJ43860, and DLGAP1 (see Online Supplemental Tables 2 and 3 for full list and details).
Discussion
In this pilot study, we have reported the novel finding that PTSD onset in military personnel is related to changes in DNA methylation as well as additional findings congruent with previous reports that DNA methylation profiles are associated with PTSD when compared to non-PTSD controls. Specifically, DNA methylation changed in participants who developed PTSD over a 3-month period in pathways related to axon guidance. In our cross-sectional analysis comparing PTSD to control subjects, a number of genes were differentially methylated, including ubiquitin-specific protease 17(USP17). These pilot findings provide novel insights into the role of epigenetics in PTSD symptoms in a young cohort of military personnel and may ultimately provide targets for future research on DNA methylation and PTSD.
Of the genes that were differentially methylated between baseline and follow-up in participants whose PTSD symptoms worsened over time, the majority (93.7%) exhibited decreased methylation including netrin-5, zinc and ring finger 3 (ZNRF3), and frizzled class receptor 9 (FZD9). Netrin-5 is of interest because members of the netrin family are essential for synaptic plasticity, primarily through the regulation of growth-cone signaling (Horn et al., 2013; Ziel & Sherwood, 2010). According to the mouse models, netrin-5 is expressed in the adult hippocampus and corpus callosum and is involved in neurogenesis (Yamagishi et al., 2015). Since PTSD is associated with hippocampal volume and function changes (Fragkaki, Thomaes, & Sijbrandij, 2016), this finding suggests that netrin-5 may be related to these pathological changes. ZNRF3, an E3 ubiquitin-protein ligase, regulates the Wnt signaling pathway by mediating the ubiquitination of Wnt receptors including receptors in the frizzled family (Jiang, Charlat, Zamponi, Yang, & Cong, 2015; Peng et al., 2013). FZD9 is one such receptor protein involved in Wnt signaling. It is crucial for hippocampal development, and its continued expression in the adult hippocampus (Zhao et al., 2005; Zhao & Pleasure, 2004) suggests that FZD9 contributes to neural plasticity. These pilot findings suggest that altered methylation patterns of genes in the Wnt signaling network may be associated with PTSD symptoms.
Our findings suggest that changes in methylation contribute to PTSD onset and implicate genes related to altered activation of axonal guidance. Axonal guidance signaling, which functions through interaction with netrin signaling and the Wnt signaling pathways, plays important roles in neural plasticity through direction of neuronal growth cones (Fortress & Frick, 2016; Guthrie, 2007; Maguschak & Ressler, 2011). Disruption to the Wnt signaling pathway has been linked to psychological disorders, including depression and anxiety (Freese, Pino, & Pleasure, 2010), and to the onset of PTSD in a preclinical model (Maguschak & Ressler, 2012). The connection between Wnt signaling and memory formation, the Wnt signaling pathway’s role in neuroplasticity, and localization of Wnt signaling molecules in the hippocampus suggest that this pathway may direct some of the neuronal changes associated with PTSD. Given the changes in neural volume observed in patients with PTSD (Morey et al., 2012), particularly in the hippocampus and amygdala, it may be that differential regulation of axonal guidance and related signaling pathways affect neurological alterations and should be explored further as a potential mechanism of PTSD pathology.
Our findings in the present pilot study also suggest that differential methylation of ubiquitin-specific proteases is associated with both increases in PTSD symptoms over time and differences between PTSD and control subjects. Ubiquitin activity is essential for the degradation of proteins that accumulate within neurons and compromise functioning and integrity. USP17, which codes for the deubiquitinating enzyme USP17 and was significantly hypermethylated in the PTSD group, regulates the nuclear function and stability of interleukin 33 (Ni et al., 2015), which is highly expressed in the brain and known to have a critical role in the pathogenesis of Alzheimer’s disease (Fu et al., 2016). Studies have also demonstrated the effect of the ubiquitin pathway in neurodegenerative diseases and brain injury, showing that sufficient regulation is essential for neuronal protection and recovery (Atkin & Paulson, 2014). Thus, the present pilot data suggest that ubiquitin pathways are implicated in PTSD and should be further investigated.
The current pilot study has inherent limitations that temper the conclusions that we can draw from these data. The sample was small and consisted of relatively young males in the military. As such, statistical limitations in the cross-sectional analysis make it difficult to confirm that differences in methylation between groups were not driven in part by comorbid conditions such as depression symptoms or self-reported TBI or confounded by individual patient characteristics. We utilized an inventory of PTSD symptoms rather than a clinical diagnosis. While the PCL-M approximates a PTSD diagnosis and allows quantification of changes in symptom severity over time, it is not synonymous with a clinical diagnosis of PTSD. MeDIP-seq does not provide high enough resolution to identify which specific CpG are methylated; instead, it provides several hundred base pair regions of DNA that contain methylated CpG. We extracted methylated DNA from whole blood to improve clinical biomarker applications; therefore, cell heterogeneity needs to be considered in future DNA methylation studies to form conclusions about specific mechanisms contributing to PTSD symptoms. Nonetheless, the longitudinal analysis we performed in the current study in conjunction with the symptom measurement is a unique strength because it allowed exploration of the association between gene methylation and changes to PTSD symptomology.
Our findings suggest that differential methylation of genes is associated with the development of PTSD symptoms over time and implicate the Wnt and axonal guidance pathways as well as ubiquitin genes. These results provide a starting point for future research to determine biomarkers of PTSD and understand the role of DNA methylation as a regulatory mechanism of PTSD.
The data in this publication have been deposited in NCBI’s Gene Expression Omnibus (GEO) and are accessible through GEO series accession number GSE93673 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE93673).
Supplemental Material
Supplemental Material, Cho_17010005_toSage_suppl_(1)(1) for Altered DNA Methylation Patterns Associated With Clinically Relevant Increases in PTSD Symptoms and PTSD Symptom Profiles in Military Personnel by Christiana Martin, Young-Eun Cho, Hyungsuk Kim, Sijung Yun, Rebekah Kanefsky, Hyunhwa Lee, Vincent Mysliwiec, Ann Cashion, and Jessica Gill in Biological Research For Nursing
Footnotes
Author Contributions: Christiana Martin and Young-Eun Cho contributed to conception, design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Hyungsuk Kim contributed to conception, design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Sijung Yun contributed to acquisition and analysis, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Rebekah Kanefsky contributed to interpretation, drafted the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Hyunhwa Lee contributed to conception and design, drafted the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Vincent Mysliwiec contributed to acquisition, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Ann Cashion contributed to interpretation, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Jessica Gill contributed to conception, design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Intramural Department of the National Institutes of Health, National Institute of Nursing Research. This study was also funded, in part, by the Center for Neuroscience and Regenerative Medicine (Grant 60855).
Supplemental Material: Supplementary material for this article is available online.
References
- Almli L. M., Fani N., Smith A. K., Ressler K. J. (2014). Genetic approaches to understanding post-traumatic stress disorder. International Journal of Neuropsychopharmacology, 17, 355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Atkin G., Paulson H. (2014). Ubiquitin pathways in neurodegenerative disease. Frontiers in Molecular Neuroscience, 7, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boks M. P., Rutten B. P., Geuze E., Haoutepen L. C., Vermetten E., Kaminsky Z., Vinkers C. H. (2016). SKA2 methylation is involved in cortisol stress reactivity and predicts the development of post-traumatic stress disorder (PTSD) after military deployment. Neuropsychopharmacology, 41, 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush S. S. (Ed.). (2014). Psychological assessment of veterans. New York, NY: Oxford University Press. [Google Scholar]
- Cesur R., Sabia J. J., Tekin E. (2013). The psychological costs of war: Military combat and mental health. Journal of Health Economics, 32, 51–65. [DOI] [PubMed] [Google Scholar]
- Fischer H. (2014). A guide to US military casualty statistics: Operation inherent resolve, operation new dawn, operation Iraqi freedom, and operation enduring freedom. Washington, DC: US Congressional Research Service. [Google Scholar]
- Fisher M. P. (2014). PTSD in the U.S. military, and the politics of prevalence. Social Science & Medicine, 115, 1–9. [DOI] [PubMed] [Google Scholar]
- Fortress A. M., Frick K. M. (2016). Hippocampal Wnt signaling: Memory regulation and hormone interactions. Neuroscientist, 22, 278–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkaki I., Thomaes K., Sijbrandij M. (2016). Posttraumatic stress disorder under ongoing threat: A review of neurobiological and neuroendocrine findings. European Journal of Psychotraumatology, 7, 30915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese J. L., Pino D., Pleasure S. J. (2010). Wnt signaling in development and disease. Neurobiology of Disease, 38, 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu A. K., Hung K. W., Yuen M. Y., Zhou X., Mak D. S., Chan I. C.…Ip N. Y. (2016). IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proceedings of the National Academy of Sciences of the U S A, 113, E2705–E2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie S. (2007). Neurotrophic factors: Are they axon guidance molecules? In Bagnard D. (Ed.), Axon growth and guidance. New York, NY: Springer, 81–94. [DOI] [PubMed] [Google Scholar]
- Horn K. E., Glasgow S. D., Gobert D., Bull S. J., Luk T., Girgis J.…Kennedy T. E. (2013). DCC expression by neurons regulates synaptic plasticity in the adult brain. Cell Reports, 3, 173–185. [DOI] [PubMed] [Google Scholar]
- Hughes K. C., Shin L. M. (2011). Functional neuroimaging studies of post-traumatic stress disorder. Expert Review of Neurotherapeutics, 11, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Charlat O., Zamponi R., Yang Y., Cong F. (2015). Dishevelled promotes Wnt receptor degradation through recruitment of ZNRF3/RNF43 E3 ubiquitin ligases. Molecular Cell, 58, 522–533. [DOI] [PubMed] [Google Scholar]
- Maguschak K. A., Ressler K. J. (2011). Wnt signaling in amygdala-dependent learning and memory. Journal of Neuroscience, 31, 13057–13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguschak K. A., Ressler K. J. (2012). A role for WNT/beta-catenin signaling in the neural mechanisms of behavior. Journal of Neuroimmune Pharmacology, 7, 763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R. A., Gold A. L., LaBar K. S., Beall S. K., Brown V. M., Haswell C. C.…McCarthy G. (2012). Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Archives of General Psychiatry, 69, 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysliwiec V., Gill J., Lee H., Baxter T., Pierce R., Barr T. L.…Roth B. J. (2013). Sleep disorders in US military personnel: A high rate of comorbid insomnia and obstructive sleep apnea. Chest, 144, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y., Tao L., Chen C., Song H., Li Z., Gao Y.…Li B. (2015). The deubiquitinase USP17 regulates the stability and nuclear function of IL-33. International Journal of Molecular Sciences, 16, 27956–27966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W. C., de Lau W., Madoori P. K., Forneris F., Granneman J. C., Clevers H., Gros P. (2013). Structures of Wnt-antagonist ZNRF3 and its complex with R-Spondin 1 and implications for signaling. PLoS One, 8, e83110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampp C., Binder E. B., Provencal N. (2014). Epigenetics in posttraumatic stress disorder. Progress in Molecular Biology and Translational Science, 128, 29–50. [DOI] [PubMed] [Google Scholar]
- Robertson K. D. (2005). DNA methylation and human disease. Nature Reviews Genetics, 6, 597–610. [DOI] [PubMed] [Google Scholar]
- Rush A. J., Trivedi M. H., Ibrahim H. M., Carmody T. J., Arnow B., Klein D. N.…Keller M. B. (2003). The 16-item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry, 54, 573–583. [DOI] [PubMed] [Google Scholar]
- Sadeh N., Spielberg J. M., Logue M. W., Wolf E. J., Smith A. K., Lusk J.…Miller M. W. (2016). SKA2 methylation is associated with decreased prefrontal cortical thickness and greater PTSD severity among trauma-exposed veterans. Molecular Psychiatry, 21, 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrio H. P., Nelson L. A., Betthauser L. M., Harwood J. E., Brenner L. A. (2011). Postdeployment traumatic brain injury screening questions: Sensitivity, specificity, and predictive values in returning soldiers. Rehabilitation Psychology, 56, 26–31. [DOI] [PubMed] [Google Scholar]
- Xue C., Ge Y., Tang B., Liu Y., Kang P., Wang M., Zhang L. (2015). A meta-analysis of risk factors for combat-related PTSD among military personnel and veterans. PLoS One, 10, e0120270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S., Yamada K., Sawada M., Nakano S., Mori N., Sawamoto K., Sato K. (2015). Netrin-5 is highly expressed in neurogenic regions of the adult brain. Frontiers in Cellular Neuroscience, 9, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., Daskalakis N. P., Desarnaud F., Makotkine I., Lehrner A. L., Kock E.…Bierer L. M. (2013). Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Frontiers in Psychiatry, 4, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., Flory J. D., Bierer L. M., Henn-Haase C., Lehrner A., Desarnaud F.…Meaney M. J. (2015). Lower methylation of glucocorticoid receptor gene promoter 1F in peripheral blood of veterans with posttraumatic stress disorder. Biological Psychiatry, 77, 356–364. [DOI] [PubMed] [Google Scholar]
- Zhao C., Aviles C., Abel R. A., Almli C. R., McQuillen P., Pleasure S. J. (2005). Hippocampal and visuospatial learning defects in mice with a deletion of frizzled 9, a gene in the Williams syndrome deletion interval. Development, 132, 2917–2927. [DOI] [PubMed] [Google Scholar]
- Zhao C., Pleasure S. J. (2004). Frizzled-9 promoter drives expression of transgenes in the medial wall of the cortex and its chief derivative the hippocampus. Genesis, 40, 32–39. [DOI] [PubMed] [Google Scholar]
- Ziel J. W., Sherwood D. R. (2010). Roles for netrin signaling outside of axon guidance: A view from the worm. Developmental Dynamics, 239, 1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Cho_17010005_toSage_suppl_(1)(1) for Altered DNA Methylation Patterns Associated With Clinically Relevant Increases in PTSD Symptoms and PTSD Symptom Profiles in Military Personnel by Christiana Martin, Young-Eun Cho, Hyungsuk Kim, Sijung Yun, Rebekah Kanefsky, Hyunhwa Lee, Vincent Mysliwiec, Ann Cashion, and Jessica Gill in Biological Research For Nursing