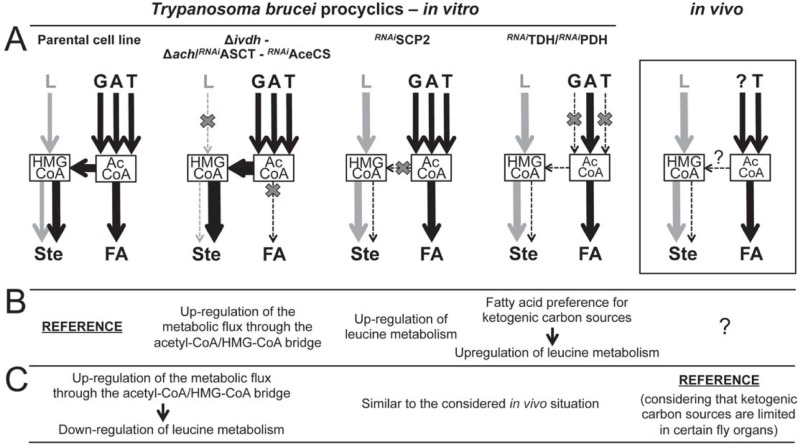
Fig 10. Metabolic flux distributions in parental and mutant cell lines.
Schemes in panel A compare metabolic flux distribution between the different branches of fatty acid and sterol biosynthesis of the T. brucei procyclic parental and mutant cell lines grown in the carbon source-rich SDM79 medium (in vitro). The carbon sources included in the model are leucine, acetate, glucose and threonine, but not fatty acids, since their incorporation into lipids through de novo biosynthetic pathways has not been demonstrated in rich medium yet. The arrow thickness reflects the strength of metabolic flux redistributions, such as upregulation of leucine metabolism and fatty acid preference, observed in the Δivdh, Δach/RNAiASCT, RNAiAceCS, RNAiSCP2 and/or RNAiTDH/RNAiPDH mutants compared to the parental PCF cell line. The estimated flux distribution in PCF trypanosomes developing in the tsetse fly midgut is presented in the right box chart. The question mark indicates that the in vivo ketogenic carbon source(s) supplementing threonine, as well as the flux through the acetyl-CoA/HMG-CoA bridge are unknown; this diagram assumes a limited availability of ketogenic carbon sources. Panel B describes metabolic adaptations using as reference the parental PCF grown in rich in vitro conditions. The question mark means that the possible metabolic adaptation in vivo is still unknown, since the carbon source contents in the tsetse's organs, including the gut and salivary glands, remain unknown. In Panel C, these metabolic adaptations are re-interpreted considering the probable physiological conditions that PCF have to face in vivo as reference, with the assumption that ketogenic carbon sources are limited in the tsetse midgut and/or in the salivary glands. Abbreviations: A, acetate; AcCoA, acetyl-CoA; FA, fatty acids; G, glucose; HMGCoA, 3-hydroxy-3-methylglutaryl-CoA; L, leucine; T, threonine; Ste, sterols.