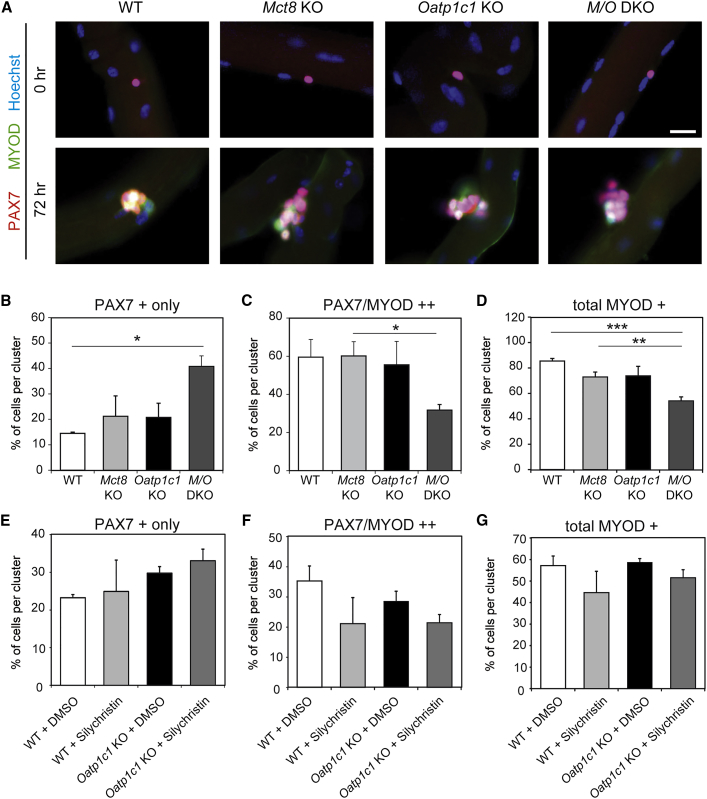
Figure 4.
SC Activation and Differentiation Is Delayed in Myofiber Cultures of M/O DKO Mice
(A) WT, Mct8-, and/or Oatp1c1-deficient primary EDL myofibers (n = 3) were either fixed directly (0 hr) or cultured for 72 hr and incubated with antibodies against PAX7 (in red) and the activation and differentiation marker MYOD (green) as well as with Hoechst33528 to label cell nuclei (in blue). MYOD immunoreactivity was only detected after 72 hr of culture as expected.
(B) Marker-positive cell nuclei were quantified and revealed a higher percentage of PAX7 only immunopositive nuclei in M/O deficiency at 72 hr.
(C–D) Both the percentage of PAX7/MYOD double-positive (C) and total MYOD-positive (D) nuclei per cluster were reduced in cultures derived from M/O DKO mice.
(E–G) EDL myofibers from WT (n = 4) and Oatp1c1 KO mice (n = 6) were cultured for 72 hr with the MCT8 inhibitor Silychristin or DMSO. Analysis of PAX7- and MYOD-immunopositive cells demonstrated a tendency toward a higher number of PAX7+ only cells (E) and a reduced number of PAX7/MYOD-immunopositive cells (F), but not toward total MYOD-positive cell numbers (G) in Silychristin-treated Oatp1c1-deficient myofibers. These findings support a delayed SC activation and differentiation if OATP1C1 and MCT8 are absent or inhibited. Group means + SEM are shown.
∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Two-way ANOVA and Bonferroni-Holm post hoc testing. Scale bar: 10 μm.