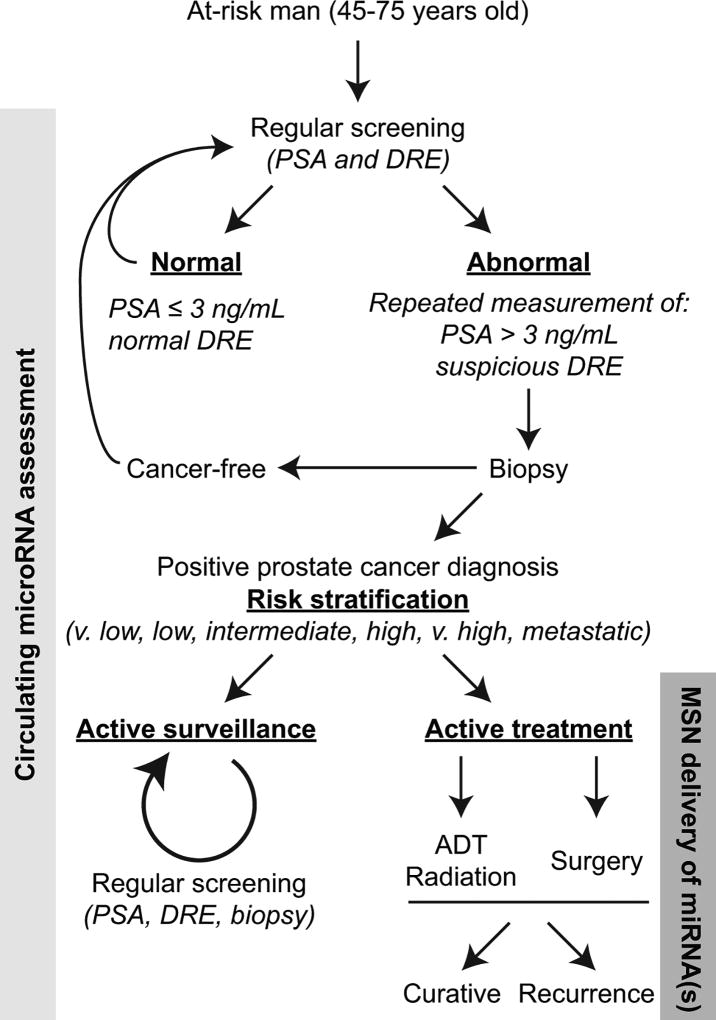
FIGURE 1.
Incorporating C-miRNAs and MSNs into prostate cancer screening and treatment. The flowchart of prostate cancer screening, diagnosis, and treatment is based on the 2017 NCCN Guideline recommendations (full version available at NCCN.org). We posit that assessment of prostate cancer associated miRNAs in circulation can complement current standard-of-care algorithms to provide personalized information on cancer cell-related activities. Targeted delivery of MSNs loaded with miRNA therapeutics specifically to prostate tumors may supplement ADT, radiation therapy, or surgery. PSA, prostate specific antigen; DRE, digital rectal exam; ADT, androgen-deprivation therapy; MSN, mesoporous silica nanoparticle