Figure 5.
Induction of Leukemia Antigen-Specific CTLs By CD40Lhigh CD4+ iPS-T Cells
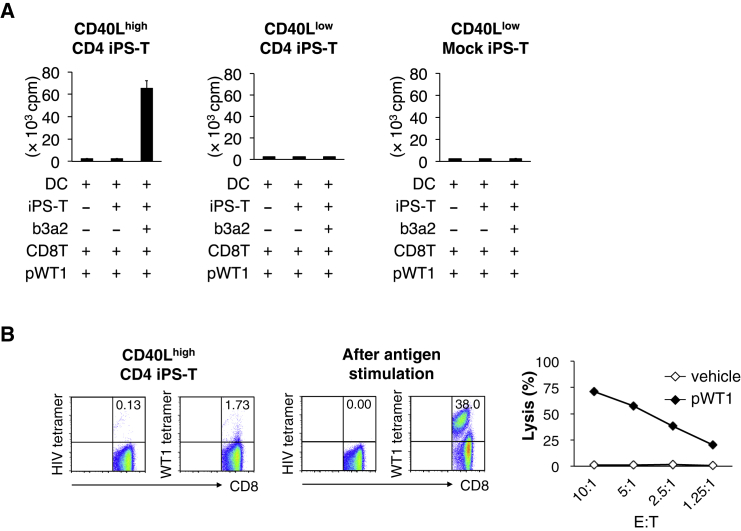
(A) Proliferative response of CD8+ T cells. Indicated iPS-T cells (5 × 103) and DCs (1 × 104) ± b3a2 peptide (5 μM) were initially co-cultured for 5 hr to mature the DCs, after which DCs and iPS-T cells were irradiated and cultured with autologous CD8+ T cells (5 × 104) in the presence of WT1 peptide (5 μM). The proliferative response (day 7) was measured as the amount of [3H]thymidine incorporated. Data shown are the means ± SD of triplicate cultures and are representative of two independent experiments.
(B) Left panels: frequency of WT1/HLA-A24 tetramer-positive CD8+ T cells primed by CD40Lhigh CD4+ iPS-T cell-conditioned DCs. CD40Lhigh CD4+ iPS-T cells (5 × 103) and DCs (1 × 104) prepulsed with b3a2 peptide (5 μM) were initially co-cultured for 5 hr to mature the DCs, after which DCs and CD4+ iPS-T cells were irradiated and cultured with autologous CD8+ T cells (5 × 104) in the presence of WT1 peptide (5 μM). Tetramer staining at day 10 after stimulation is shown. Center panel: frequency of WT1/HLA-A24 tetramer-positive CD8+ T cells after a third stimulation with WT1 peptide. Representative flow cytometry profiles of two independent experiments. HIV-env/HLA-A24 tetramer was used as a control. Right panel: cytotoxic activities of expanded WT1-specific CD8+ T cells against K562-A24 loaded with vehicle or WT1 peptide. Cytotoxicity was measured by 51Cr-release assay for 4 hr at the indicated E:T ratios. Data are representative of two independent triplicate experiments.