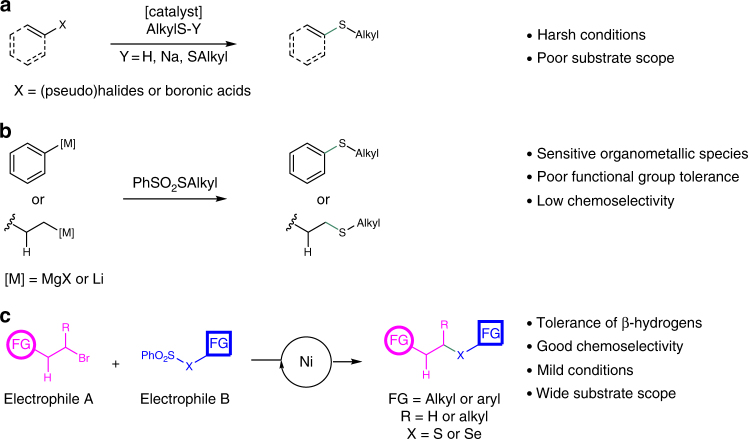
Fig. 1.
Unsymmetrical alkyl-thiolation through different processes. a In traditional methods, transition-metal-catalyzed coupling of activated Csp2–X with alkyl thiols were well developed, however, harsh reaction conditions are usually required. b Compared to traditional thiolation reagents, thiosulfonates are easily prepared and bench-stable, which can react with Grignard reagents or organolithium reagents to generate corresponding alkyl sulfides. c Nickel-catalyzed reductive thiolation of unactivated alkyl bromides with thiosulfonates as reliable thiolation reagents was described. This reaction is easy to handle, scalable, and proceeds smoothly with excellent tolerance of functional groups