Table 3.
Substrate scope for nickel-catalyzed reductive alkyl-thiolation and selenylation
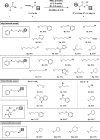
|
Reaction conditions A: for X = S, alkyl bromides 1 (1.1 equiv.), benzenesulfonothioates 2 (1.0 equiv.), NiBr2 (5.0 mol%), L5 (7.5 mol%), Mn (1.5 equiv.), DMF/MeCN (v/v = 2:3, 0.2 M), N2 atmosphere, 100 °C, 12 h; reaction conditions B: for X = Se, alkyl bromides 1 (1.1 equiv.), benzenesulfonoselenoates 2 (1.0 equiv.), NiBr2 (5.0 mol%), L5 (7.5 mol%), Mn (1.5 equiv.), DMF (0.2 M), N2 atmosphere, 30 °C, 12 h. Yields of isolated products are given
†80 °C, 16 h
‡1 (0.3 mmol), 2 (0.33 mmol), 80 °C, 16 h
#1 (3 equiv.) was used, 80 °C
*1 (3 equiv.) was used
