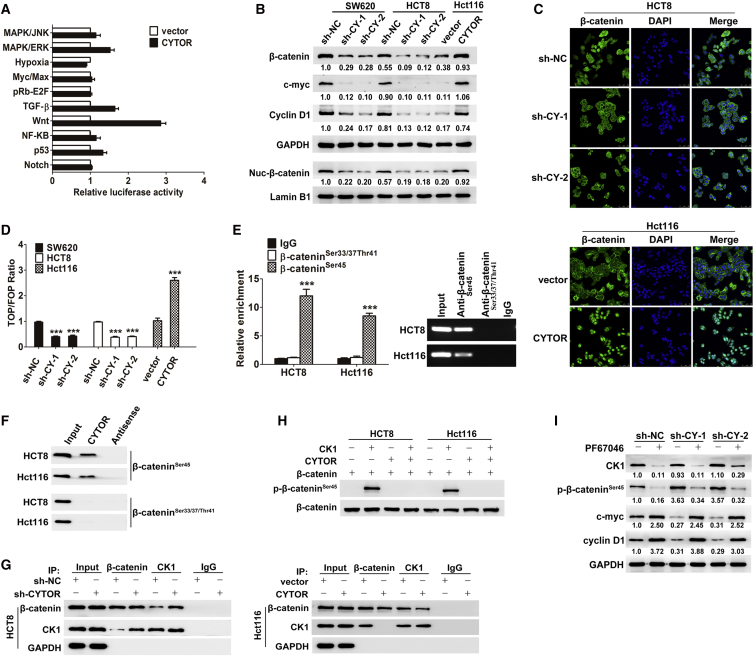
Figure 3.
CYTOR Activates the Wnt/β-Catenin Signaling by Blocking CK1-Mediated β-Catenin Phosphorylation
(A) Signaling pathway reporter array was used for seeking the relative pathway associated with CYTOR on Hct116 cells. (B) Knockdown of CYTOR caused a decreased expression of total β-catenin, nuclear β-catenin, c-myc, and cyclin D1, whereas overexpression of CYTOR significantly increased their expression. (C) The role of CYTOR in promoting the redistribution of cytoplasmic β-catenin to nuclear localization was confirmed through immunofluorescence assays. Depletion of CYTOR resulted in a redistribution of nuclear β-catenin to the cytoplasmic localization, whereas overexpression of CYOTR caused substantial nuclear accumulation of β-catenin. (D) TOPflash and FOPflash luciferase reporter analyses revealed that the transactivation of TCF reporter was inhibited by the depletion of CYTOR and enhanced via overexpression of CYTOR. (E and F) RIP assay (E) and RNA pull-down assay (F) revealed that non-phospho-β-cateninSer45, rather than other active non-phospho sites, was required for β-catenin interaction with CYTOR. (G) Coimmunoprecipitation of CK1 and β-catenin in lysates of colon cancer cells with modified CYTOR expression. (H) The role of CYTOR in protecting β-catenin from phosphorylation by CK1 was confirmed via an in vitro phosphorylation assay. (I) HCT8 cells were treated with 10 nmol/L PF670462 for 24 hr, inhibiting CK1 activity by PF670462 abolished the effects of CYTOR-shRNA on β-cateninSer45 phosphorylation and Wnt/β-catenin signaling activity. Error bars, ±SD. ***p < 0.001.