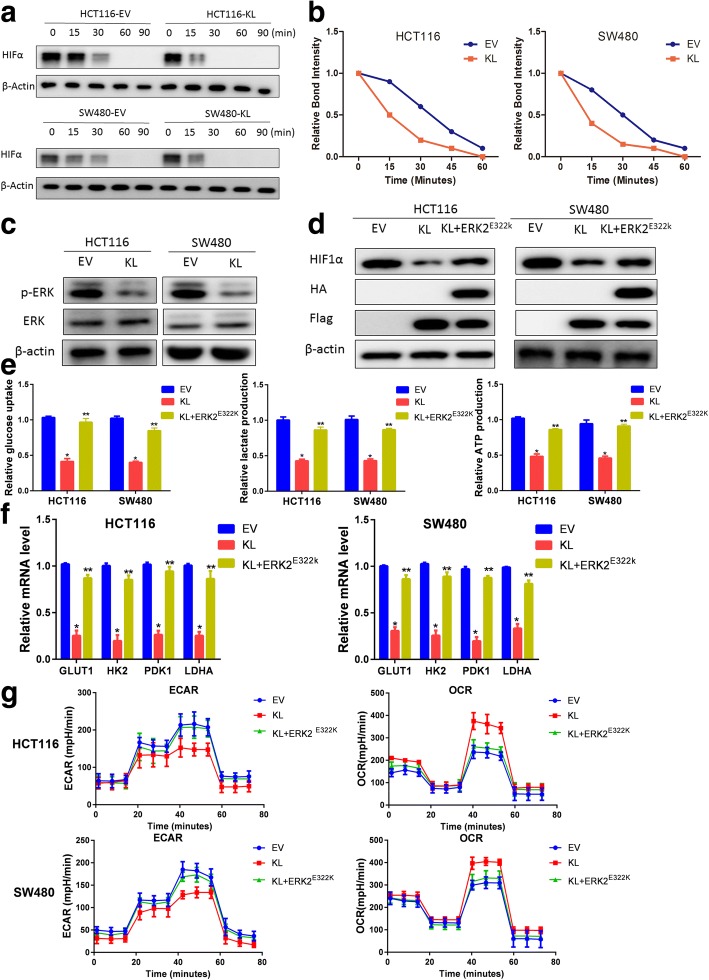
Fig. 5.
KL regulates HIF1α in an ERK dependent manner. Based on the observation that KL correlated with HIF1α in protein level instead of mRNA level, we assumed that KL might regulate HIF1α stability. First, we examined the hall-life of HIF1α in KL overexpressed HCT-116 and SW480 cells, and results indicated that KL significantly decreased the half-life of HIF1α (a and b). To seek the underlying molecular mechanism, we examined the activation status of ERK1/2, which regulated HIF1α protein level. In KL overexpressed colon cancer cells, the activation of ERK1/2 was inhibited, indicating that KL might regulate HIF1α via ERK signaling pathway (c). To answer whether KL regulated HIF1α via ERK signaling pathway, we overexpressed constitutive activation mutant of ERK2 (ERK2E322K) in KL overexpressed cells, and results demonstrated that ERK2E322K introduction could alleviate the decrease in HIF1α caused by KL (d). Subsequently, we examined the effect of ERK2E322K on glycolysis in KL overexpressed cells and found ERK2E322K transfection could eliminate the inhibit effect of KL on glycolysis (e). Real-time PCR analysis of HIF1α targeted glycolysis genes supported this hypothesis, as ERK2E322K introduction could partially up-regulate the mRNA level of glycolysis genes in KL overexpressed colon cancer cells (f). Then, we performed glycolysis analysis, and found that ERK2E322K introduction could induce an increase in ECAR in KL-overexpressed colon cancer cells. In the end, the OCR analysis demonstrated that ERK2E322K introduction could alleviated the attenuation in OCR caused by KL introduction (g). Taken together, these results indicated that KL regulated aerobic glycolysis via ERK1/2 activation. *P < 0.05, ** P > 0.05