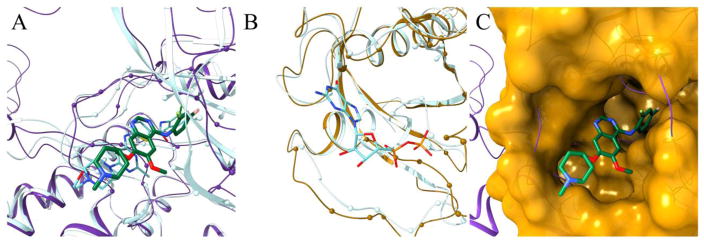
Figure 3.
Repositioning of vandetanib from protein-tyrosine kinase 6 (PTK6) to GTPase KRas (KRAS) according to eRepo-ORP. PTK6 and KRAS proteins are colored purple and gold, respectively, whereas ligands are colored by atom type (green/teal – carbon, blue – nitrogen, red – oxygen, yellow – sulfur, citron – chlorine, pink – fluorine, cyan – bromine). (A) Structure model of the complex between PTK6 (purple ribbons) and vandetanib (thick sticks) with predicted binding residues shown as spheres superposed onto the experimental structure of PTK6 (teal ribbons) bound to dasatinib (thin sticks). (B) Structure model of KRAS (gold ribbons) with predicted drug-binding residues shown as spheres superposed onto the experimental structure of KRAS (teal ribbons) bound to ADP (thin sticks). (C) Local superposition of PTK6 (purple ribbons) and KRAS (gold surface) according to the sequence order-independent pocket alignment by eMatchSite. Annotated binding residues in KRAS are solid, whereas the remaining surface is transparent. Vandetanib repositioned to KRAS is represented by thick sticks.