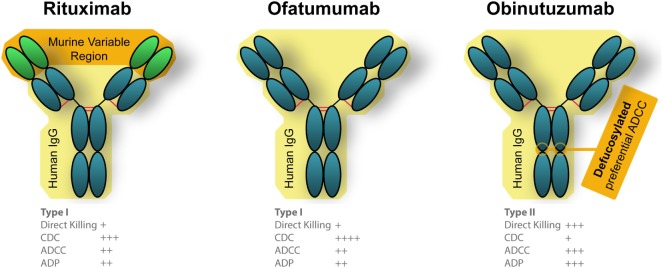
Figure 3.
Engineered differences between Food and Drug Administration approved anti-CD20 monoclonal antibodies (mAbs). Rituximab is a chimeric mAb that is partially humanized, that has a human Fc portion but retains the murine variable region which recognizes CD20. Both ofatumumab and obinutuzumab are fully humanized mAbs, which reduces unintended immune responses against the therapies. Ofatumumab also has a glycoengineered Fc region which results in better binding with immune effector cells (106, 190).