Figure 1.
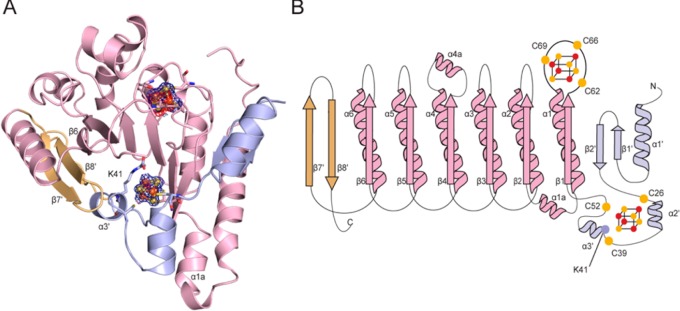
Overall architecture of TYW1. (A) TYW1 adopts a partial (β/α)6 TIM barrel fold (light pink), which is expanded at both the N-terminus (light blue) and the C-terminus (light orange). The radical SAM cluster and auxiliary [4Fe–4S] cluster are shown as spheres (iron in orange and sulfur in yellow) surrounded by 2Fo–Fc electron density contoured at +1 σ (blue). When the radical SAM cluster is refined as a [4Fe–4S] cluster, negative difference density (−3 σ, red) is visible, suggesting either incomplete assembly of the cluster or partial cluster degradation. The location of K41 is also shown. (B) Topology diagram of the overall structure of TYW1, colored as in part A. The cysteine ligands to the clusters are shown as yellow spheres. The position of K41 is represented as a blue sphere.