Abstract
Background
Allogeneic hematopoietic stem cell transplant patients are at risk of invasive fungal infections and prophylaxis with azole agents is common practice. The concomitant use of these agents with sirolimus and tacrolimus for the prevention of graft-versus-host disease may result in excessive immunosuppression or toxicity.
Methods
This retrospective study identified hospitalized patients who underwent allogeneic hematopoietic stem cell transplantation between August 2009 and April 2011 at Rush University Medical Center. From this group, patients who underwent concomitant tacrolimus, sirolimus, and azole therapy were included for evaluation. The immunosuppression dosing in conjunction with azole use at discharge was analyzed to develop a dosing algorithm dependent on whether fluconazole, posaconazole, or voriconazole was used.
Results
A total of 36 patients were screened for inclusion, of which 8 were excluded due to acute renal failure and/or hemolysis. The remaining patients were stratified by the azole they were concomitantly taking with tacrolimus and sirolimus. The fluconazole arm required the lowest magnitude of dose reductions, while voriconazole required the greatest.
Conclusion
Dose reductions of 50–75% for both sirolimus and tacrolimus, in combination with standard dosing of azole antifungal agents, were necessary to achieve therapeutic drug concentrations for immunosuppressants and potentially avoid toxicities.
Keywords: Sirolimus, tacrolimus, azoles, hematopoietic stem cell transplantation, interaction, dosing scheme
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is frequently complicated by acute graft-versus-host disease (GVHD).1,2 Acute GVHD results from the immunologic attack of donor cytotoxic T cells and inflammatory cytokines mounted against the recipient and results in target organ damage, most commonly affecting the skin, liver, and gastrointestinal tract. Prevalence of acute GVHD ranges from 35% to 80%, and severe, grade IV GVHD is associated with a 5-year mortality of 95%.3 The complications of GVHD warrant the use of immunosuppressive therapy to reduce its incidence and decrease morbidity and mortality.
A phase II trial has shown the combination of sir-olimus and tacrolimus to be an effective immunosuppressive regimen as prophylaxis for GVHD in allo-HSCT.4,5 One of the consequences of acute GVHD is the risk of secondary infections, including invasive fungal infections (IFIs) associated with transplantation. Patients undergoing allo-HSCT are susceptible to IFIs because of prolonged periods of neutropenia, severe mucositis, and the presence of a central venous catheter.6–8 Prospective, randomized, double-blind studies comparing prophylactic therapy with fluconazole versus placebo have shown significant reductions in the incidence of IFIs.9,10 Since these studies, azole agents with broader antifungal coverage have been developed and used as preventative agents.11–13 The National Comprehensive Cancer Network and the Infectious Diseases Society of America recognize azole antifungal agents as prophylaxis for IFIs.14,15
Simultaneous use of sirolimus, tacrolimus, and azole antifungals results in significant, well documented, pharmacokinetic interactions and potential for drug toxicities.16–20 The sharing of common metabolic pathways leads to elevated serum concentrations of immunosuppressants and toxicity from supratherapeutic drug levels. Current literature addresses dose adjustments for the use of azoles with sirolimus or tacrolimus as individual agents.21,22 However, the literature does not address dosage adjustments when using sirolimus, tacrolimus, and an azole concurrently for GVHD and IFI prophylactic therapy. Current dose reductions at Rush University Medical Center (RUMC) are based on anecdotal evidence and professional opinion from available literature with a single interacting agent, and further adjustments are based on immunosuppressant levels. Prior to discharge, a goal for allo-HSCT patients initiated on sirolimus and tacrolimus at RUMC is attainment of a dosing regimen that yields therapeutic serum trough concentrations. Therefore, it would be advantageous to develop an algorithm that would yield therapeutic levels in a time efficient manner.
The objective of our study was to construct dosing algorithms for concomitant use of immunosuppressants, specifically with tacrolimus and sirolimus, and azole antifungal therapy for the prophylaxis of GVHD and IFIs for patients undergoing allo-HSCT. We hypothesized that the optimal dosing regimen for tacrolimus and sirolimus will differ based on the prophylactic azole used.
Materials and methods
RUMC is a 676-bed, tertiary care academic medical center in Chicago, Illinois. The Section of Stem Cell Transplant and Cell Therapy perform roughly 100 transplantations annually, of which approximately 25% are allo-HSCTs. The study was approved by the hospital's Institutional Review Board and carried out from August 2009 through April 2011. The start date marks the implementation of the sirolimus and tacrolimus immunosuppressive regimen at RUMC.
This retrospective chart review included hospitalized patients who underwent allo-HSCT during the study period, as identified from an internal electronic hematopoietic transplant patient database. From this group, all patients who underwent concurrent sirolimus, tacrolimus, and azole therapy were included for evaluation. Patients were excluded if they were not on the aforementioned agents at the time of discharge. The electronic medical record system at RUMC was used to collect data, including: patient age, gender, ethnicity, height, weight, hematologic malignancy, stem cell donor type, serum creatinine (SCr), hepatic function, initiation day of prophylactic therapy, azole regimen, and use of other concurrent interacting medications. Interacting medications were identified during data collection and included: aminoglycosides, amphotericin B liposomal, phenytoin, diltiazem, selective serotonin reuptake inhibitors, and ganciclovir. In regards to sir-olimus and tacrolimus, data collection focused on serum trough concentrations and initial and discharge doses.
Immunosuppressive therapy is initiated as tacrolimus 0.02 mg/kg/day intravenously by continuous infusion (target serum trough concentration, 5–10 ng/mL) and sirolimus 12 mg oral loading dose followed by 4mg orally daily (target serum trough concentration, 3–12 ng/mL) on day −3 before transplant.4 Dosing of immunosuppressants is determined via a modified adjusted body weight equation. The internal “dosing weight” is calculated as follows: (0.5 × (actual body weight – ideal body weight) + ideal body weight). RUMC utilizes a conversion of intravenous tacrolimus to an equipotent oral dose prior to discharge at a conservative one to three ratio.
Antifungal therapy is initiated as fluconazole 400 mg daily intravenously or orally on day −3. If warranted by the clinical scenario or active infection, antifungal therapy may be modified prior to discharge to either posaconazole 600 mg/day orally in three equally divided doses, prophylactic voriconazole 400 mg/day orally in two equally divided doses, or treatment voriconazole 4 mg/kg/day orally in two equally divided doses. Therapeutic drug monitoring of azoles in the allo-HSCT population was not performed during the time of this study. In the event that azole therapy was modified during the hospital stay, then the azole at steady-state concentrations at discharge determined patient stratification in the development of the dosing algorithm.
Acute renal failure and hemolysis were defined subjectively in our study and was dependent upon diagnoses in the electronic medical record at the time of immunosuppressive regimen modification. Therefore, only excluded patients were able to receive a diagnosis of acute renal failure or hemolysis. Steady-state was defined as continuation of therapy for five biological half-lives of a given drug after initiation or dose adjustment.
Descriptive statistics, linear correlation, and analysis of variance (ANOVA) was performed using SPSS version 14.0 software.
Results
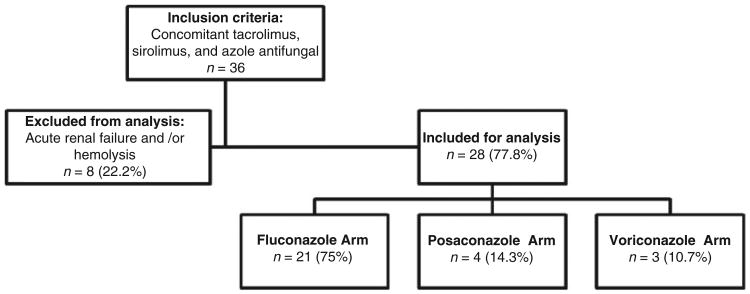
A total of 36 patients were screened for inclusion, of which 8 (22%) were excluded. All eight of the excluded patients had acute renal failure (baseline SCr of 1.5 mg/dL or less) and three had hemolysis. Five of the excluded patients were taking fluconazole and three were taking voriconazole. Four of eight excluded patients had sirolimus initiated at a lower dose than outlined per protocol (range, 50–87.5%). The remaining 28 patients were stratified by the azole they were concomitantly taking with sirolimus and tacrolimus (Figure 1).
Figure 1.
Stratification by azole antifungal.
The demographic and baseline clinical characteristics are provided in Table 1. Two patients were classified as unknown race due to incompleteness or involuntary patient response in the electronic medical record. Of 28, 19 (67.9%) patients received a matched related allo-HSCT. In the posaconazole and voriconazole arms, no patients had lymphoma as their underlying malignancy, and no patients received a mismatched allo-HSCT. The mean SCr peak during hospital stay reached three times baseline and two times baseline in the posaconazole and voriconazole arms, respectively. Hepatic function, acutely measured by alanine aminotransferase (ALT) and aspartate aminotransferase (AST), reached the upper limits of normal in all three study arms during hospital stay but normalized prior to discharge.
Table 1.
Baseline patient demographics.
| Variable | Fluconazole | Posaconazole | Voriconazole |
|---|---|---|---|
| Sample size | 21 | 4 | 3 |
| Age in years, median (range) | 47 (24–58) | 50 (32–61) | 21 (20–65) |
| Sex, number (%) | |||
| Male | 13 (61.9) | 2 (50.0) | 1 (33.3) |
| Female | 8 (38.1) | 2 (50.0) | 2 (66.7) |
| Race, number (%) | |||
| Caucasian | 13 (61.9) | 3 (75.0) | 2 (66.7) |
| Hispanic | 5 (23.8) | 1 (25.0) | – |
| Asian | 1 (4.8) | – | 1 (33.3) |
| Unknown | 2 (9.5) | – | – |
| Hematologic malignancy, number (%) | |||
| Acute lymphoblastic leukemia | 4 (19.0) | – | 1 (33.3) |
| Acute myeloid leukemia | 9 (42.9) | 2 (50.0) | 1 (33.3) |
| Chronic myeloid leukemia | 1 (4.8) | 1 (25.0) | – |
| Non-Hodgkin lymphoma | 2 (9.5) | – | – |
| Hodgkin lymphoma | 3 (14.3) | – | – |
| Myelofibrosis | 2 (9.5) | 1 (25.0) | – |
| Myelodysplastic syndrome | – | – | 1 (33.3) |
| Stem-cell donor type, number (%) | |||
| Matched unrelated | 3 (14.3) | 1 (25.0) | 2 (66.7) |
| Matched related | 15 (71.4) | 3 (75.0) | 1 (33.3) |
| Mismatched unrelated | 2 (9.5) | – | – |
| Mismatched related | 1 (4.8) | – | – |
| Renal function, mean ± SD | |||
| SCr on admission (mg/dL) | 0.9 ± 0.2 | 1.0 ± 0.2 | 0.8 ± 0.3 |
| SCr on discharge (mg/dL) | 1.0 ± 0.3 | 1.3 ± 0.3 | 1.6 ± 1.5 |
| SCr peak (mg/dL) | 1.1 ± 0.4 | 3.1 ± 3.1 | 1.6 ± 1.5 |
| Hepatic function, mean ± SD | |||
| ALT on admission (U/L) | 29 ± 24 | 29 ± 34 | 26 ± 17 |
| ALT on discharge (U/L) | 29 ± 25 | 24 ± 13 | 17 ± 6 |
| ALT peak (U/L) | 47 ± 35 | 38 ± 14 | 48 ± 43 |
| AST on admission (U/L) | 21 ± 12 | 20 ± 12 | 26 ± 13 |
| AST on discharge (U/L) | 30 17 | 30 20 | 21 4 |
| AST peak (U/L) | 43 ± 22 | 44 ± 14 | 49 ± 27 |
| Length of stay post-transplant, mean ± SD | 25 ± 5 | 17 ± 3 | 32 ± 21 |
Sirolimus and tacrolimus trough concentrations at discharge for all azoles were within therapeutic range 89.3–60.7% of the time, respectively (Tables 2 and 3). Seven (33.3%) patients in the fluconazole arm had sir-olimus initiated at a dose 50% lower than outlined per protocol. In the posaconazole arm, two patients had sirolimus initiated at doses lower than outlined per protocol of 50% and 87.5%. The latter also had a concomitant tacrolimus dose reduction of 25% on initiation. In the voriconazole arm, two patients had sirolimus initiated at doses lower than outlined per protocol of 50% and 90%. The latter also had a concomitant tacrolimus dose reduction of 70% on initiation.
Table 2.
Sirolimus data by azole.
| Variable | Fluconazole | Posaconazole | Voriconazole | All azoles |
|---|---|---|---|---|
| Highest recorded trough (ng/mL), mean ± SD | 17.2 ± 5.4 | 14.4 ± 5.3 | 10.2 ± 4.2 | – |
| Trough on discharge (ng/mL), mean ± SD | 7.8 ± 2.8 | 5.7 ± 2.8 | 7.3 ± 5.4 | – |
| Trough on discharge, median (range) | 7.1 (4.1–14.3) | 5.1 (3.1–9.6) | 8.7 (2.8–13.5) | – |
| Therapeutic (goal 3–12 ng/mL), yes (%) | 20 (95.2) | 4 (100) | 1 (33.3) | 25 (89.3) |
| Maintenance oral dose at discharge (mg/day), mean ± SD | 1.7 ± 1.1 | 1.3 ± 0.5 | 1.2 ± 0.8 | – |
Table 3.
Tacrolimus data by azole.
| Variable | Fluconazole | Posaconazole | Voriconazole | All azoles |
|---|---|---|---|---|
| Highest recorded trough (ng/mL), mean ± SD | 15.8 ± 10.7 | 16.5 ± 12.5 | 10.0 ± 2.9 | – |
| Trough on discharge (ng/mL), mean ± SD | 8.0 ± 2.6 | 6.1 ± 2.0 | 8.3 ± 4.1 | – |
| Trough on discharge, median (range) | 8.8 (3.5–11.7) | 6.0 (4.2–8.3) | 7.1 (5.0–12.9) | – |
| Therapeutic (goal 5–10 ng/mL), yes (%) | 12 (57.1) | 3 (75.0) | 2 (66.7) | 17 (60.7) |
| Maintenance oral dose at dischargea (mg/kg/day), mean ± SD | 0.038 ± 0.024 | 0.038 ± 0.020 | 0.018 ± 0.011 | – |
Based on dosing weight of (0.5 × (actual body weight – ideal body weight) + ideal body weight).
In the fluconazole arm, one patient was initially started on fluconazole 400 mg/day, but was decreased to 200 mg/day secondary to decreased renal function. All patients on posaconazole received a dose of 600 mg/day in three equally divided doses. Two patients in the voriconazole arm received the drug at 400 mg/day in two equally divided doses, while one patient received 8 mg/kg/day, calculated to be 700 mg/day, in two equally divided doses. Three and one patients in the posaconazole and voriconazole arms were transitioned from initial fluconazole, respectively.
Linear regression and ANOVA analyses were only performed for the fluconazole arm. Linear regression analysis was performed using the following variables at the time of discharge: age, SCr, AST, ALT, immuno-suppressant doses, and concomitant utilization of interacting drugs. No statistically significant correlation was identified. Furthermore, ANOVA analysis was performed using the following variables: sex, race, hematologic malignancy, stem-cell donor type, and immunosuppressant doses at discharge.
Discussion
This retrospective study reports the results of concomitant administration of tacrolimus, sirolimus, and azole therapy, with the objective of developing a dosing algorithm. To our knowledge, no prior study has addressed dosing adjustments when using all three of these interacting agents concomitantly.
The development of our algorithm is based on the assumption that patients would have therapeutic concentrations of their immunosuppressants at discharge. Sirolimus and tacrolimus trough concentrations on discharge were within therapeutic range 89.3% and 60.7% of the time, respectively (Tables 2 and 3). It is not surprising that sirolimus concentrations were therapeutic more frequently because the therapeutic range for tacrolimus in the outlined protocol is narrower. The lowest and highest single trough concentration on discharge for tacrolimus was 3.5 and 12.9 ng/mL, respectively. We assumed that this range of sub- and supratherapeutic concentrations at discharge may not be clinically significant and have calculated our dosing recommendations with inclusion of these patients.
Our recommendations for starting doses, provided in Table 4, were formulated from the average maintenance oral doses at hospital discharge. The recommendations do not take into account the initial sirolimus loading dose and product availability. A sirolimus loading dose three times greater than our recommended maintenance doses would reflect the bolus to maintenance dose ratio used in our study. Clinicians will need to weigh the practicality of our recommendations for each patient and round doses to available strengths and formulations of each drug.
Table 4.
Recommended starting dosing algorithm for concomitant administration of tacrolimus, sirolimus, and an azole.
| Variable | Fluconazole | Posaconazole | Voriconazole |
|---|---|---|---|
| Sirolimus (mg/day), oral | 1.7 | 1.3 | 1.2 |
| Tacrolimus (mg/kg/day), intravenous | 0.01 | 0.01 | 0.005 |
| Tacrolimus (mg/kg/day), oral | 0.038 | 0.038 | 0.018 |
The summary of our final dosing recommendations reiterates that interactions between agents are multifactorial, taking place at the cytochrome P450 (CYP) 3A4 isoenzyme and with P-glycoprotein (P-gp). Enzyme inhibition at CYP 3A4 may occur after administration of a single dose of an inhibitor drug.22 However, given the long half-lives of azoles, a maximal effect may not be seen until steady-state concentrations are typically reached after 5–7 days.19,21 The variability in our calculated dose reductions between azoles is apparent, with voriconazole requiring the greatest reduction and fluconazole the least. Our spectrum of recommended dose adjustments falls in line with previous reported literature concerning the potency of individual azoles (Table 5).
Table 5.
| Cytochrome P450 3A4 | P-glycoprotein | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Drug | Substrate | Inhibitor | Potency | Substrate | Inhibitor |
| Fluconazole | Yes | Yes | + | Yes | No |
| Posaconazole | ND | Yes | ++to+++ | Yes | Yes |
| Voriconazole | Yes | Yes | +++ | No | No |
| Sirolimus | Yes | ND | ND | Yes | Yes |
| Tacrolimus | Yes | ND | ND | Yes | Yes |
ND: no data.
Due to the retrospective design of this study, the efficacy of this algorithm remains unstudied. We would contend that our algorithm is the best available data for guiding administration of these agents concomitantly, but is not a substitute for clinical judgment. Further caution should be taken when utilizing this regimen as not to subject patients to subtherapeutic concentrations of immunosuppression. Aggressive monitoring at the initiation of therapy is recommended because our algorithm has not been examined prospectively. Further prospective studies are needed to validate the benefits of our recommendations and to determine if rapidly achieving therapeutic concentrations of sirolimus and tacrolimus may reduce nephrotoxicity, hemolysis, and decrease hospital length of stay.
Admittedly, the sample sizes of our posaconazole and voriconazole arms are lacking. Patients at our institution are not given posaconazole or voriconazole as first-line agents as prophylaxis for IFIs, making it difficult to include patients. The higher sample size in the fluconazole arm makes it likely that our associated conclusions are of greater reliability than the other arms. For these reasons, we only performed linear regression and ANOVA analyses for the fluconazole arm.
Transient decreases in renal function were seen in our included patients. The posaconazole arm of our study had the largest increase in SCr during treatment. It is unclear whether this may be a cause or result of having the highest mean recorded trough level of tacrolimus, a known nephrotoxic agent. The voriconazole arm also had an increase in SCr during hospital stay, but the mean of the highest serum trough concentration recorded for immunosuppressants was the lowest of the three arms. We attribute this result to the fact that all patients in the voriconazole arm had immunosuppressant doses initiated at least 50% less than outlined per protocol.
While we did not aim to study safety or side effects of combining these agents, we did find remarkable data. As the study progressed, doses of immunosuppressants were initiated at doses lower than outlined by the protocol at the discretion of the clinician in an effort to prevent toxicity secondary to routine supratherapeutic levels. Despite these efforts, our study still excluded 8 of 36 patients who developed acute renal failure and/ or hemolysis. We did not extend data collection to include resolution or progression of toxicities. Given that four of eight excluded patients did have dose reductions of their initial immunosuppressive regimen and still experienced toxicity, we suggest that these dose reductions may not have been of appropriate magnitude for each individual agent.
Limitations with this study are built around the ability of clinicians to subjectively initiate immunosuppressants at doses lower than outlined per protocol and broaden antifungal therapy. Anecdotal dose reductions of immunosuppressive therapy on initiation became more frequent as the study progressed and revealed reactive responses to therapy.
Our dosing algorithm only focused on discharge doses and immunosuppressant serum trough concentrations. Initial doses of immunosuppressive therapy and transition between azoles were not pertinent in the development of our dosing algorithm since serum concentrations of sirolimus and tacrolimus were therapeutic and steady-state of azole therapy was reached prior to discharge. On the contrary, one may argue that duration of exposure to the drug–drug interaction may be relevant in predicting toxicity, but was not addressed by our study. Given these limitations, we recommend further study with prospective implementation of our algorithm.
Conclusion
The optimal dosing regimen for concomitant use of immunosuppression, specifically with tacrolimus and sirolimus, and azole antifungal therapy for the prophylaxis of GVHD and IFIs for patients undergoing allo-HSCT is dependent upon the azole used. Dose reductions of 50–75% for both sirolimus and tacrolimus, in combination with standard dosing of azole antifungal agents, were necessary to achieve therapeutic drug concentrations for immunosuppressants and potentially avoid toxicities. A high degree of acute renal failure and hemolysis is seen when combining these agents; and therefore, further prospective study of our dosing recommendations is needed to validate whether these toxicities may be avoided through early attainment of therapeutic sirolimus and tacrolimus concentrations.
Acknowledgments
The authors acknowledge Mimi Lo, Deborah Hass, and Sunita Nathan for assistance in formulating the study proposal. We are grateful for the work of Christopher Crank, who provided statistical support and analysis.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest: None declared.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Weisdorf D. GVHD the nuts and bolts. Hematol Am Soc Hematol Educ Program. 2007;2007:62–67. doi: 10.1182/asheducation-2007.1.62. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutler C, Kim HT, Hochberg E, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:328–336. doi: 10.1016/j.bbmt.2003.12.305. [DOI] [PubMed] [Google Scholar]
- 5.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes PD, Marr KA. Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br J Haematol. 2007;139:519–531. doi: 10.1111/j.1365-2141.2007.06812.x. [DOI] [PubMed] [Google Scholar]
- 7.Michallet M, Ito JI. Approaches to the management of invasive fungal infections in hematologic malignancy and hematopoietic cell transplantation. J Clin Oncol. 2009;27:3398–3409. doi: 10.1200/JCO.2008.20.1178. [DOI] [PubMed] [Google Scholar]
- 8.Prentice HG, Kibbler CC, Prentice AG. Towards a targeted, risk-based, antifungal strategy in neutropenic patients. Br J Haematol. 2000;110:273–284. doi: 10.1046/j.1365-2141.2000.02014.x. [DOI] [PubMed] [Google Scholar]
- 9.Goodman JL, Winston DJ, Greenfield RA. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 10.Slavin MA, Osborne B, Adams R. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation-a prospective, randomized, double-blind study. J Infect Dis. 1995;171:1545–1552. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 11.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 12.Ulmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 13.Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111–5118. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baden LR, Bensinger W, Angarone M, et al. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw. 2012;10:1412–1445. doi: 10.6004/jnccn.2012.0146. [DOI] [PubMed] [Google Scholar]
- 15.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cervelli MJ. Fluconazole-sirolimus drug interaction. Transplantation. 2002;74:1477–1478. doi: 10.1097/00007890-200211270-00024. [DOI] [PubMed] [Google Scholar]
- 17.Marty FM, Lowry CM, Cutler CS, et al. Voriconazole and sirolimus coadministration after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:552–559. doi: 10.1016/j.bbmt.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 18.Sádaba B, Campanero MA, Quetglas EG, et al. Clinical relevance of sirolimus drug interactions in transplant patients. Transplant Proc. 2004;36:3226–3228. doi: 10.1016/j.transproceed.2004.10.056. [DOI] [PubMed] [Google Scholar]
- 19.Trifilio SM, Scheetz MH, Pi J, et al. Tacrolimus use in adult allogeneic stem cell transplant recipients receiving voriconazole: preemptive dose modification and therapeutic drug monitoring. Bone Marrow Transplant. 2010;45:1352–1356. doi: 10.1038/bmt.2009.345. [DOI] [PubMed] [Google Scholar]
- 20.Uberti JP, Cronin S, Ratanatharathorn V. Optimum use of tacrolimus in the prophylaxis of graft versus host disease. BioDrugs. 1999;11:343–358. doi: 10.2165/00063030-199911050-00006. [DOI] [PubMed] [Google Scholar]
- 21.Dodds-Ashley E. Management of drug and food interactions with azole antifungal agents in transplant recipients. Pharmacotherapy. 2010;30:842–854. doi: 10.1592/phco.30.8.842. [DOI] [PubMed] [Google Scholar]
- 22.Saad AH, DePestel DD, Carver PL. Factors influencing the magnitude and clinical significance of drug interactions between azole antifungals and select immunosuppressants. Pharmacotherapy. 2006;26:1730–1744. doi: 10.1592/phco.26.12.1730. [DOI] [PubMed] [Google Scholar]