Scheme 3.
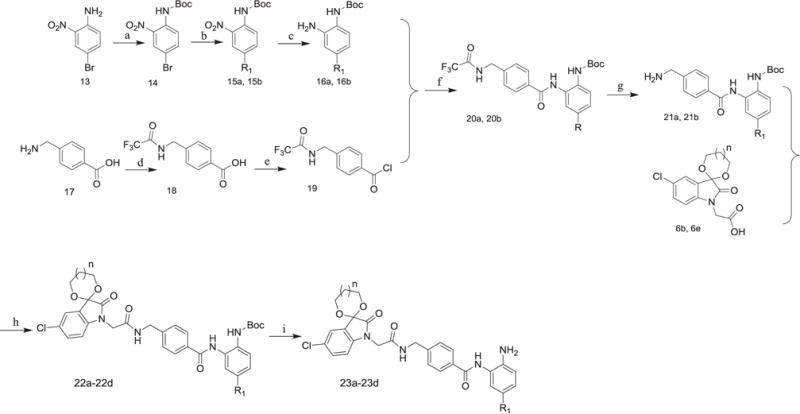
(1) R1 = thienyl or phenyl. (2) Reagents and conditions: (a) (Boc)2O, Et3N, DMAP, CH2Cl2, r.t.; (b) thiophene-2-boronic acid or phenylboronic acid, Pd[P(pH3)4], Na2CO3, 1, 4-dioxane, H2O, 90 °C; (c) Pd/C, H2, CH3OH/EtOAc, r.t.; (d) (CF3CO)2O, 0 °C; (e) SOCl2, THF; reflux; (f) pyridine, r.t.; (g) K2CO3, H2O, THF/CH3OH, 70 °C; (h) o-phenylenediamine, TBTU, Et3N, THF/CH2Cl2, r.t.; (i) EtOAc/HCl, r.t.
