Figure 5. ZNFX-1 forms stable complex with an RdRP, EGO-1, and argonautes in both gene-silencing and gene-activating pathways.
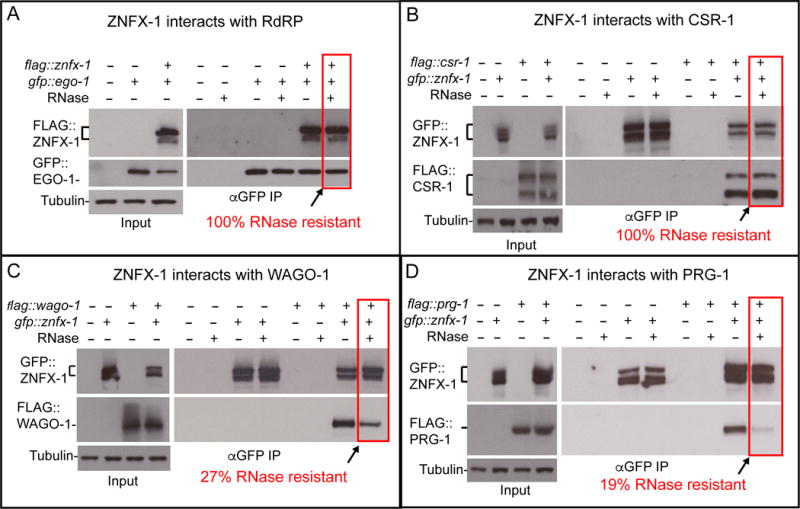
(A-D) Co-immunoprecipitation experiments showing physical interaction between ZNFX-1 and EGO-1 (A), CSR-1 (B), WAGO-1(C) or PRG-1(D). Presence (+) or absence (-) of the tagged proteins or RNase I treatment are indicated. Immunoprecipitation was performed using α-GFP antibody, and the blots were probed with α -GFP or α -flag antibodies, and with α -tubulin antibody for loading control. Brackets indicates the positions of two major isoforms of znfx-1 and csr-1. % of RNase resistant complex as determined by the relative amounts of proteins in ZNFX-1 immunoprecipiates with or without the RNase treatment, are indicated. Level of co-precipitating protein was normalized to the amount of ZNFX-1 in the immunoprecipitate.
