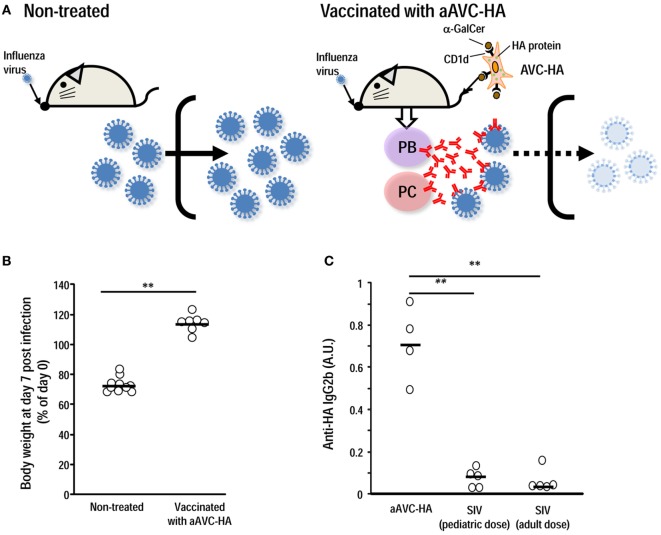
Figure 2.
Vaccination with aAVC-HA protects against influenza virus infection. (A) Mice were initially vaccinated with 5 × 105 aAVC-HA. Two weeks later, non-treated and the aAVC-HA vaccinated mice were challenged with a lethal dose of PR8 influenza virus to assess production of virus-neutralizing antibody. (B) Non-treated and vaccinated mice were evaluated for weight loss a week after an infection. (C) HA-specific antibody in the serum was assessed by ELISA 2 weeks after administration of three types of vaccines to C57BL/6 mice, two standard doses of the standard influenza vaccine (0.75 µg/kg, the human pediatric dose, and 0.3 µg/kg, the human adult dose) or the aAVC-HA (mean ± SEM, n = 4–7) **P < 0.01.