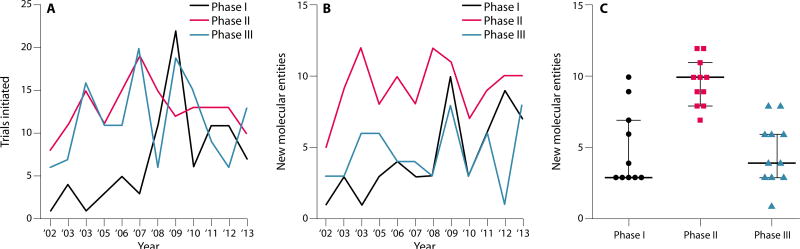
Fig. 3. The drug pipeline in PD.
(A) The number of interventional phase I trials (black) for new molecular entities that are disease-modifying or that alleviate symptoms shows a trend upwards over the last 10 years. The number of phase II (red, mean = 12.9 SD = 2.8) and phase III (blue, mean = 11.58 SD = 5.0) trials has remained stable. (B) The number of new molecular entities entering interventional PD trials each year. Phase I (mean = 4.7 SD = 2.8), phase II (mean = 9.6 SD = 1.6), phase III (mean = 4.7 SD = 2.2). (C) Ten-year average for new molecular entities in phase I, phase II and phase III trials. Data are from www.clinicaltrials.gov. Interventional (symptom or disease-modifying) PD clinical trials using a drug, biologic, or gene therapy were selected; PET molecular imaging studies were excluded.