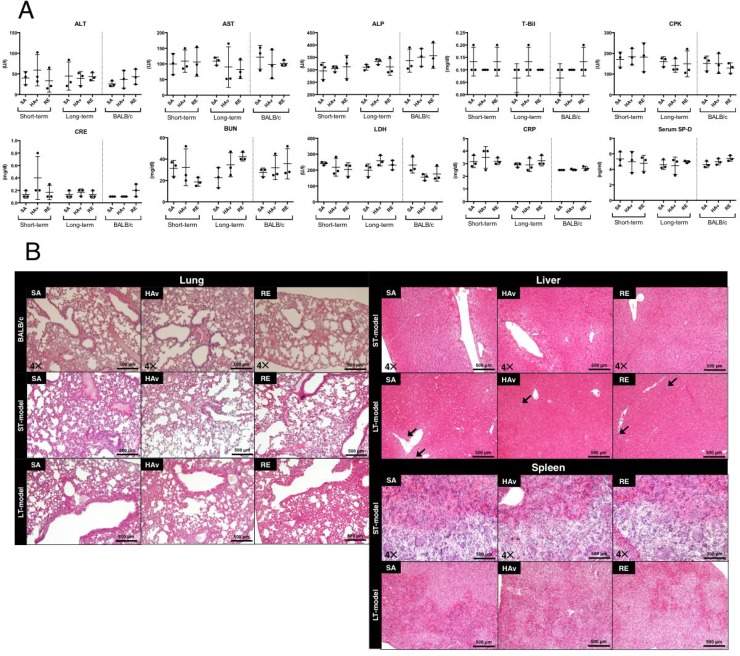
Figure 2. Blood biochemical data and lung, liver, and spleen histopathology of the short-term and long-term humanized mouse models and BALB/c mice.
Each animal group was inoculated with the toxicity reference vaccine (RE), hemagglutinin split vaccine (HAv), or saline (SA), and 16 h after the vaccination, serum was collected to obtain blood biochemical data on blood urea nitrogen (BUN), creatinine (CRE), creatinine kinase (CPK), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (T-Bil), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), C-reactive protein (CRP), and serum surfactant protein-D (SP-D) (A). The lungs, liver, and spleen were collected for the histopathological examination (B). Multiple 4-μm-thick slices were stained with hematoxylin and eosin (H&E). The arrows indicate infiltration by leukocytes.