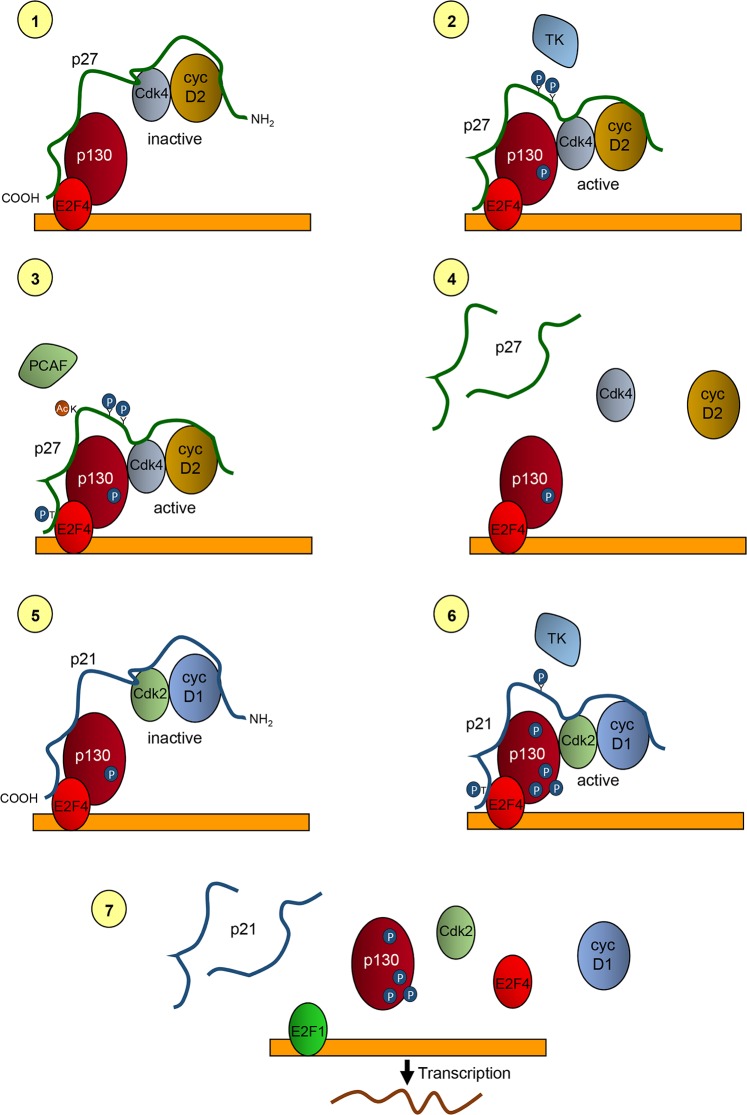
Figure 5. Model of the role of p27 and p21 in the transcriptional regulation of p130/E2F4 repressed genes.
In quiescent cells p27 is associated with p130 and E2F4 on the promoters of target genes by its COOH- moiety. It is also bound to cyclin D2/3 and Cdk4 by is NH2-half. At this stage, Cdk4 is inactive due to its inhibition by p27 (1). At early-mid G1 phase of the cell cycle p27 is phosphorylated by tyrosine kinases at residues Y74 and Y88. These phosphorylations allow retrieving Cdk activity and thus Cdk4 can phosphorylate p130 (2). Moreover, Cdk4 phosphorylate T187 of p27 facilitating its degradation. Degradation is also stimulated by the acetylation of K100 by the acetyltransferase and transcriptional co-activator PCAF (3). Degradation of p27 leads to the removal of cyclin D2/3 and Cdk4 from the promoters (4). After that, p21 associates with p130/E2F4 by its carboxyl terminus and recruits to the promoters cyclin D1-Cdk2 complexes that are associated with its NH2-moiety (5). After phosphorylation of the Y77 (in human) of p21, Cdk2 becomes activated and able to multi-phosphorylate p130. Cdk2 also phosphorylates S130 of p21 leading to its degradation (6). Phosphorylation of p130 and degradation of p21 disrupt the repressive complexes thus, allowing the action of activator transcription factors as E2F1 that initiate transcription of target genes (7).