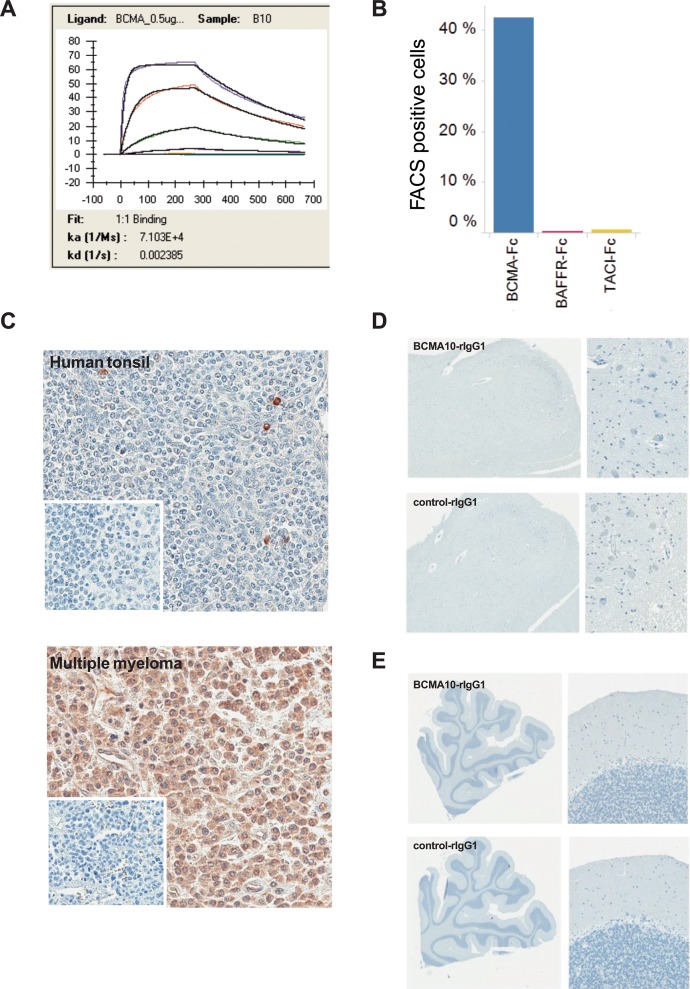
Figure 5. Evaluation of binding specificity and affinity of the clone 10 scFv.
(A) Biacore T200 SPR sensogram for the interaction between clone 10 scFv and recombinant human BCMA. A purified recombinant His-tagged protein containing the scFv of clone 10 was used to determine the binding affinity to recombinant BCMA protein. hBCMA-Fc was immobilized on the chip surface via biotin:streptavidin interaction and clone 10 scFv was flowed over the chip at 1:3 dilutions. Shown are the association constant (ka) and disassociation constant (kd) determined after fitting to a 1:1 binding model used to determine the apparent affinity to rhBCMA-Fc. (B) CAR clone 10 was transduced into Jurkat cells and incubated with recombinant Fc-tagged BCMA, BAFFR, or TACI to assess the binding of these proteins to the cell surface-expressed CAR. Binding was detected using an anti-Fc antibody by flow cytometry. The percent of cells with a fluorescence level above the untransduced Jurkat cells is shown. (C) IHC staining of human tissue with the clone 10 chimeric antibody demonstrates scattered positive cells in the normal human tonsil (grade 1) and intense uniform staining of multiple myeloma tissue (grade 5) (insert: irrelevant antibody negative control). (D) IHC staining of normal human medulla oblongata with a chimeric antibody containing the clone 10 scFv. (E) IHC staining of normal cerebellum with a chimeric antibody contain the clone 10 scFv.