Abstract
BACKGROUND
The definition of frailty, as modeled by the Fried criteria, has been limited primarily to the physical domain. The purpose of this study was to assess the additive value of cognitive function with existing frailty criteria to predict poor postoperative outcomes in a large multidisciplinary cohort of patients undergoing major operations.
STUDY DESIGN
A 4-level composite frailty scoring system was created via the combination of the Fried frailty score and the Emory Clock Draw Test to assess preoperative frailty and cognitive impairment, respectively. Overall survival was defined as months from date of operation to date of death or last follow-up.
RESULTS
This study included 330 patients undergoing major operations; mean age was 58 years and a total of 53 patient deaths occurred during 4-year follow-up. Among the robust cohort, 20 of 168 patients died (11.9%), and among those who were both physically frail and cognitively impaired, 11 of 26 patients died (42.3%). Multivariable analysis demonstrated the physically frail and cognitively impaired cohort to have a 3.92 higher risk of death (95% CI 1.66 to 9.26) compared with the cohort of robust patients (p = 0.002). Kaplan-Meier survival curves reveal an overall difference in long-term survival (log-rank p < 0.0001), driven mainly by the high risk of mortality among patients with both physical frailty and cognitive impairment.
CONCLUSIONS
The use of a combined frailty and cognitive assessment score has a more powerful potential to predict adult patients at higher risk of overall survival than either measurement alone. The addition of cognitive assessment to physical frailty measure can lead to improved preoperative decision making and possibly early intervention, as well as more accurate patient counseling.
Traditional surgical risk assessments are inherently subjective1,2 or simply a tabulation of a patient’s chronic diseases or comorbidities,3 leading many researchers to investigate more objective preoperative screening tools that can better identify patients who are most at risk for poor outcomes. Fried’s4 conceptualization of frailty includes a “global phenotype” of decreased physiologic reserve, leaving those affected unable to respond to external stressors and more susceptible to poor outcomes. Extended to the surgical arena, objective measures of frailty based on the Fried frailty criteria are shown to be strong predictors of poor postoperative, outcomes such as hospital readmission, mortality, and complications.4–8 In contrast to the subjective surgical risk assessments used currently,1–3 the Fried frailty score was found to be a reliable objective method of quantifying a patient’s fitness for surgery and can be a crucial aid in improving surgical decision making and counseling patients on risk.8
Currently used models of frailty, such as Fried’s4 conceptualization, focus primarily on the physical domain and do not include assessment of cognition. However, surgery is a complex process that is not only a physical stressor, but also a mental stressor.9 Many recent studies have considered incorporating the concept of cognition in the definition of frailty,10–12 and a definition for cognitive frailty has emerged. In 2013, an International Consensus Group13 on cognitive frailty was organized to propose a heterogeneous clinical manifestation characterized by the simultaneous presence of both physical frailty and cognitive impairment in the elderly.
We hypothesized that preoperative cognitive impairment in combination with frailty would be associated with poor postoperative survival outcomes in a large multidisciplinary cohort of adult patients, aged 18 years and older, undergoing major operations.
METHODS
Study design and participants
The Emory University IRB approved this prospective study of patients undergoing major surgical interventions for urologic, thoracic, neurologic, vascular, cardiovascular, transplant, general surgical, and surgical oncology illnesses at the Emory University Hospital. Inclusion criteria consisted of patients aged 18 years or older who were being evaluated for a surgical procedure requiring hospital admission, not including endoscopic procedures. Exclusion criteria consisted of an inability to ambulate, poor manual dexterity or inability to grip, and inability to read or verbally understand the questionnaires.
Physical frailty via the Fried frailty criteria
Preoperative assessment of physical frailty included the 5 components of the Fried frailty score (shrinking, weakness, exhaustion, low activity, and slowed walking speed) (eTable 1). This calculation is similar to calculations in previously studied reports on 30-day morbidity and 1-year mortality from the same institution.5,8 Each of the 5 domains of the Fried frailty score yields a dichotomous score of 0 or 1 and was summed to give a composite score. Patients were classified as not frail (0 to 1), intermediate frail (2 to 3), and frail (4 to 5). For our analysis, the intermediate frail and frail patients were combined into 1 group, given the small population of frail patients (66 patients [20%]) compared with the non-frail group (264 patients [80%]).
Cognitive impairment via the Clock Draw Test
The Clock Draw Test (CDT) provides a brief assessment of cognitive function compared with other assessments, such as the Montreal Cognitive Assessment and Mini-Mental State Exam.14 Evidence suggests the CDT is both a sensitive and specific tool for detecting moderate to severe cognitive impairment.15 Additionally, the CDT is less influenced by educational status, requires less language ability, and is not as impacted by cognitive reserve.16 The CDT primarily assesses executive function and visuospatial cognition.17–19 Ultimately, the CDT was feasible to administer by any member of the medical ancillary staff in a busy real-world surgical clinic.
During preoperative assessment, the CDT was administered according to standard instructions. The patient was presented with a predrawn circle on a piece of paper and told, “I want you to imagine that this circle is the face of a clock. Place all the numbers on the clock and set the time to ten minutes after eleven.” Initially, we included 2 different CDT scoring protocols: the Emory Alzheimer’s Disease Research Center method per Steenland and colleagues14 and the Seattle method per Lessig and colleagues.15 As the 2 scoring protocols were highly correlated (κ-statistic 0.93), we presented only results according to the Emory Alzheimer’s Disease Research Center scoring methodology. The following 3 criteria defined a normal CDT: all numbers were present with no omissions, duplications, or superfluous markings; the numbers 12, 3, 6, and 9 were on or adjacent to the appropriate quadrant, were upright, and in the predrawn circle near the edge; and the clock hands indicated 11:10 by placement and proportion. If any one of the criteria was not met, the CDT was deemed abnormal (Table 1).
Table 1.
Emory Alzheimer’s Disease Research Center Scoring Method
| No. of points | Explanation |
|---|---|
| 1 | All numbers present with no omissions, duplications, or superfluous markings. |
| 1 | The numbers 12, 3, 6, 9 were on or adjacent to the appropriate quadrant boundaries. All numbers were upright in the predrawn circle near the edge. |
| 1 | The clock hands indicated 11:10 by placement and proportion. Scorer could tell the intended time without knowing what time the participant was told to draw. |
Clock Draw Test was worth 3 points. Score of 0 to 2 was considered abnormal and a score of 3 was normal. If any 1 of the criteria was not met, the Clock Draw Test was deemed abnormal.
Composite frailty scoring system
Cognitively impaired patients were indicated by an abnormal CDT score, and non-cognitively impaired patients were indicated by a normal CDT score. A 4-level composite frailty scoring system was created via the combination of the CDT score and Fried frailty score to assess its association with clinical outcomes. Robust patients included patients who were non-frail and non-cognitively impaired, Cognitively Impaired patients included those who were only cognitively impaired and non-frail, Physically Frail patients included those who were frail and non-cognitively impaired, and Physical and Cognitively Impaired patients included patients with the simultaneous presence of both physical frailty and cognitive impairment (Table 2).
Table 2.
Composite Frailty Scoring System Cohort Classification and Abbreviation
| Abbreviation | Cohort name | Cohort classification |
|---|---|---|
| RP | Robust Patients | Frailty score 0 to 1 (non-frail) + normal Clock Draw Test score (no cognitive impairment) |
| CI | Cognitive Impairment Only | Frailty score 0 to 1 (non-frail) + abnormal Clock Draw Test score (cognitively impaired) |
| PF | Physical Frailty Only | Frailty score 2 to 5 (frail) + normal Clock Draw Test score (no cognitive impairment) |
| PFCI | Both Physical Frailty and Cognitive Impairment | Frailty score 2 to 5 (frail) + abnormal Clock Draw Test score (cognitively impaired) |
Preoperative covariates
For each patient, we collected the following clinical and demographic variables: age at time of enrollment, race, sex, BMI, and indication for operation (malignancy or other). We also assessed the American Society of Anesthesiologists classification,1 the Charlson Comorbidity Index,3 the Eastern Cooperative Oncology Group performance status,2 the Minnesota Leisure Time Activities Questionnaire,20 and common preoperative serum biochemical measurements (albumin, hemoglobin, creatinine, estimated glomerular filtration rate, and platelets).
Primary outcomes
The primary outcomes of interest were mortality and overall survival. Mortality was defined as the date of death at 30 days, 90 days, 1 year, and more than 1 year after operation. Mortality information was acquired for all patients by gathering data from the Social Security National Death Index, medical records, and follow-up phone calls. Overall survival end point was defined as months from date of operation to date of death or last follow-up per medical records and phone calls.
Statistical analyses
Statistical analyses were conducted using SAS, version 9.4 (SAS Institute), SAS macros (SAS Institute), and software developed at the Biostatistics and Bioinformatics at Emory Rollins School of Public Health. The significance level was set at 0.05. Descriptive statistics for each variable were reported for the overall population and stratified by each composite frailty cohort. The univariate association of categorical outcomes or categorical cohorts was carried out using chi-square or Fisher’s exact test for categorical covariates and ANOVA for numerical covariates. In our analysis, physical frailty and cognitive impairment were tested with all outcomes variables individually, as well as with the composite frailty score system.
Multivariable models were created for primary outcomes through logistic regression for binary outcomes and a Cox proportional hazard model for overall survival. The multiple logistic regression model was built following a backward elimination at α level of 0.3, which was produced by including all covariates initially, and then consecutively removing the variable with the largest p value > 0.3. This was continued one at a time until all covariates in the model had a p value < 0.3. Kaplan-Meier plots were generated to evaluate overall survival after operation based on the composite frailty score system.
RESULTS
Patient demographics
There were 330 patients enrolled after surgical consultation who consented to proceed with the operation. All patients were included in analysis and follow-up data were obtained for overall survival analysis. Clinical and demographic data are presented in Table 3. Mean age of patients was 58.0 years (range 18 to 89 years), and more than half of the patients were male (54.2%) and Caucasian (67.3%). Mean BMI was 28.40 kg/m2 (range 16.4 to 46.6 kg/m2). The most common procedures included 71 nephrectomy cases (21.5%), 29 radical prostatectomy cases (8.8%), and 25 radical cystectomy cases (7.6%). The indication for operation was malignancy for 216 patients (65.5%). In respect to traditional preoperative variables, most patients had an American Society of Anesthesiologist classification of 3 to 5 (73.1%) and an Eastern Cooperative Oncology Group score of 0 to 1 (96.4%). Mean Charlson Comorbidity Index for patients was 2.87 ± 2.15.
Table 3.
Demographic and Clinical Characteristics (n = 330)
| Characteristic | Data |
|---|---|
| Age, y, mean (range) | 58.0 (18–89) |
| Younger than 65 y, n (%) | 195 (59.1) |
| 65 y or older, n (%) | 135 (40.9) |
| Sex, male, n (%) | 179 (54.2) |
| Race, Caucasian, n (%) | 222 (67.3) |
| BMI, kg/m2, mean (range) | 28.40 (16.4–46.6) |
| Surgery for malignancy, n (%) | 216 (65.5) |
| ASA score, n (%) | |
| 1 to 2 | 89 (26.9) |
| 3 to 5 | 241 (73.1) |
| ECOG, n (%) | |
| 0 to 1 | 318 (96.4) |
| ≥2 | 12 (3.6) |
| CCI, mean ± SD | 2.86 ± 2.15 |
| Albumin, g/dL, mean ± SD* | 3.82 ± 0.56 |
| Hemoglobin, g/dL, mean ± SD† | 13.00 ± 2.03 |
| Creatinine, mg/dL, mean ± SD‡ | 1.44 ± 2.18 |
| eGFR, mL/min/1.73 m2, mean ± SD§ | 59 ± 15 |
| Platelets, × 103/mcL, mean ± SD‖ | 241.97 ± 89.94 |
| Frailty per Fried frailty criteria, n (%) | |
| Frail (score 0 to 1) | 264 (80.0) |
| Intermediate frail + frail (score 2 to 5) | 66 (20.0) |
| Abnormal Clock Draw Test, n (%) | |
| Abnormal Clock Draw Test (score 0 to 2)¶ | 122 (37.0) |
Serum albumin missing, n = 76.
Serum hemoglobin missing, n = 14.
Serum creatinine missing, n = 18.
eGFR missing, n = 28.
Platelets missing, n = 13.
Emory Alzheimer’s Disease Research Center clock draw (score 0 to 3; abnormal 0 to 2, normal 3).
ASA, American Society of Anesthesiologists; CCI, Charlson Comorbidity Index; ECOG, Eastern Cooperative Oncology Group; eGFR, estimated glomerular filtration rate.
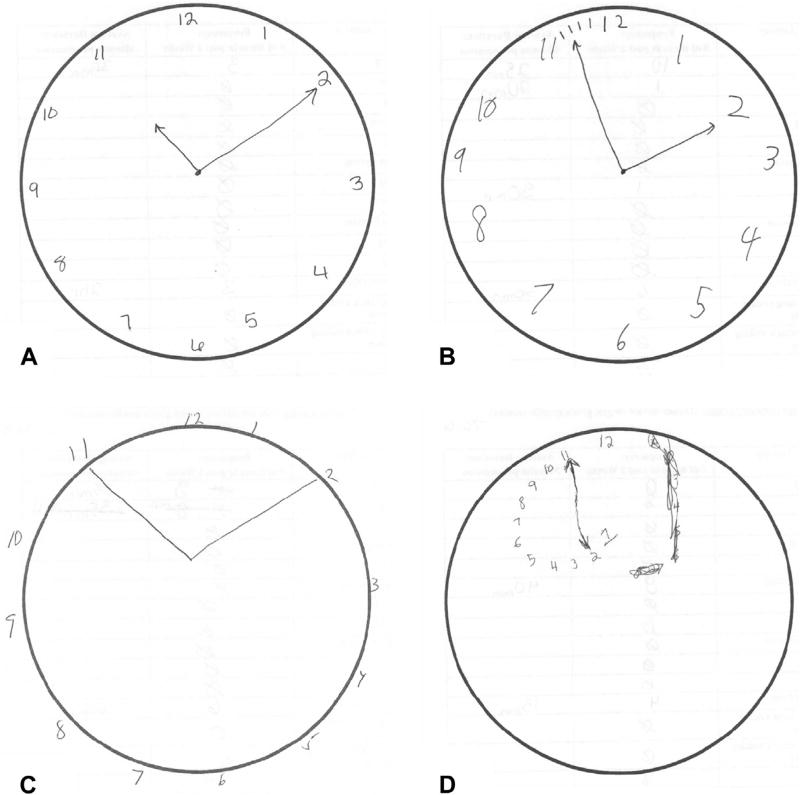
As assessed by the Fried frailty score, 264 patients (80.0%) of the total population were non-frail, with a score of 0 to 1. For our analysis, 66 patients (20.0%) of the total population were considered frail, with a score of 2 to 5; of which 63 patients were intermediate frail, with a score of 2 to 3; and 3 patients were frail with a score of 4 to 5. There were 122 patients (37.0%) with cognitive impairment based on an abnormal CDT and, importantly, half (50.8%) were younger than 65 years old. Figure 1 demonstrates an illustration of normal vs abnormal CDT scores.
Figure 1.
Sample Clock Draw Tests of patients with the following Clock Draw Test scores: (A) score of 3 (normal), (B) score of 2 (abnormal), (C) score of 1 (abnormal), and (D) score of 0 (abnormal). Emory clock draw score scale: score 0 to 3; abnormal 0 to 2, normal 3.
Independent analysis of Clock Draw Test and frailty
Univariate analysis revealed no significant association between CDT score and Fried frailty score (p = 0.648). Additionally, there was no univariate association of 30-day (p = 0.630), 90-day (p = 1.000), or 1-year (p = 0.465) mortality with the CDT score. As expected, the Fried frailty score was univariately associated with 30-day (p = 0.027), 90-day (p = 0.007), and 1-year (p = 0.003) mortality. Univariate association of overall survival during 4-year follow-up revealed a hazard ratio of 2.79 (95% CI 1.61 to 4.86; p < 0.001) for frail patients when compared with the non-frail.
Composite frailty cohort characteristics
The composite frailty scoring system identified 4 exclusive patient cohorts based on the combination of the Fried frailty score and CDT score. One hundred and sixty-eight patients (50.9%) were classified as Robust patients; 96 (29.1%) as Cognitively Impaired only; 40 (12.1%) as Physically Frail only; and 26 patients (7.9%) as Physically Frail and Cognitively Impaired. The Physically Frail and Cognitively Impaired cohort was significantly older, mostly African American, and had a higher Eastern Cooperative Oncology Group score. Demographics stratified by composite frailty scoring system are presented in Table 4.
Table 4.
Demographic and Clinical Characteristics Stratified by the Composite Frailty Scoring System
| Variable | Robust (n = 168) |
Cognitive Impairment only (n = 96) |
Physical Frailty only (n = 40) |
Physical Frailty + Cognitive Impairment (n = 26) |
p Value |
|---|---|---|---|---|---|
| Categorical age | 0.006* | ||||
| Age 65 y or older, n (%) | 57 (33.9) | 42 (43.8) | 18 (45.0) | 18 (69.2) | |
| Sex | 0.446 | ||||
| Male, n (%) | 93 (55.4) | 55 (57.3) | 17 (42.5) | 14 (53.9) | |
| Race | 0.008* | ||||
| Caucasian, n (%) | 125 (74.4) | 54 (56.3) | 29 (72.5) | 14 (53.9) | |
| BMI, kg/m2, mean (range) | 28.5 (18.0–45.1) | 29.0 (19.6–46.6) | 27.8 (16.4–41.7) | 26.6 (17.6–42.6) | 0.300 |
| ASA score, n (%) | 0.117 | ||||
| 1 to 2 | 51 (30.4) | 28 (29.2) | 6 (15.0) | 4 (15.4) | |
| 3 to 5 | 117 (69.6) | 68 (70.8) | 34 (85.0) | 22 (84.6) | |
| ECOG, n (%) | 0.011* | ||||
| 0 to 1 | 164 (97.6) | 93 (96.9) | 39 (97.5) | 22 (84.6) | |
| ≥2 | 4 (2.4) | 3 (3.1) | 1 (2.5) | 4 (15.4) | |
| CCI, n (%) | 0.864 | ||||
| 0 to 1 | 40 (23.8) | 22 (22.9) | 7 (17.5) | 6 (23.1) | |
| ≥2 | 128 (76.2) | 74 (77.1) | 33 (82.5) | 20 (76.9) | |
| Surgery for malignancy, n (%) | 105 (62.5) | 67 (69.8) | 26 (65.0) | 18 (69.2) | 0.656 |
Statistically significant.
ASA, American Society of Anesthesiologists; CCI, Charlson Comorbidity Index; ECOG, Eastern Cooperative Oncology Group.
Composite frailty and mortality
Median follow-up of living patients was 969 days (interquartile range, 465 days), and median follow-up of deceased patients was 489 days (interquartile range, 511 days). At 1 year post operation, a total of 20 patients (6.5%) had died. At 2 and 3 years of follow-up, the number of deaths had increased to 38 (14.0%) and 50 (36.2%), respectively. At the end of clinical follow-up for this study, there were a total of 53 deaths (16.1%) in our population.
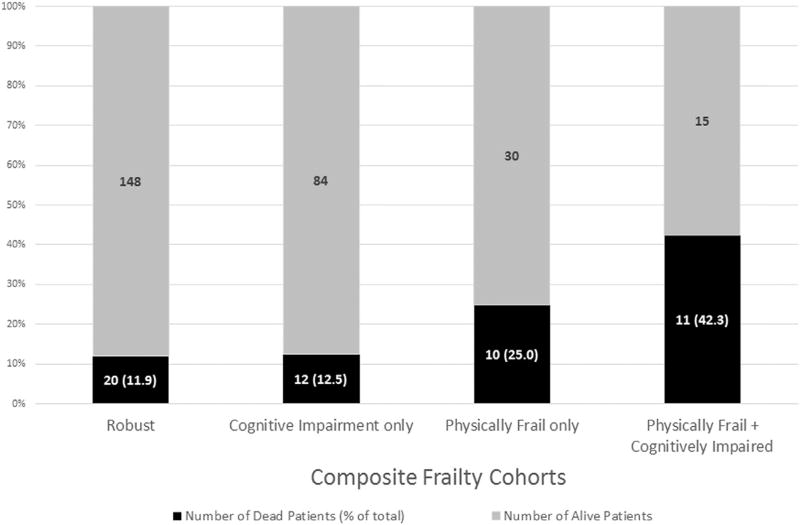
Figure 2 demonstrates patient deaths stratified by the composite frailty scoring system. During the course of the study, of 168 Robust patients, there were 20 (11.9%) deaths. Of 96 Cognitively Impaired only patients, there were 12 (12.5%) deaths. Of the Physically Frail patients, 10 of 40 (25.0%) patients without cognitive impairment died. However, when cognition was taken into consideration, 11 of the 26, or 42.3%, of the physically frail patients with cognitive impairment died, an approximately 17.3% increase from the Physically Frail only patients.
Figure 2.
Total number of deaths stratified by the composite frailty scoring system during a 4-year follow-up period.
Specifically within the first 2 years after operation, the Physically Frail and Cognitively Impaired cohort not only had the lowest overall survival rates compared with the reference group, but also had a substantial drop in survival (from 80.3% at 1 year post operation, to 54.9% at 2 years post operation) (Table 5). The number of deaths more than doubled within the Physically Frail and Cognitively Impaired cohort from 19.7% to 45.1% from 2 to 3 years after operation. In contrast, the Robust patients had a 94.3% survival rate at 1 year post operation; at 2 and 3 years after operation, survival rates decreased to 91.4% and 82.6%, respectively.
Table 5.
Mortality Stratified by the Composite Frailty Scoring System
| Variable | Robust (n = 168) |
Cognitive Impairment only (n = 96) |
Physical Frailty only (n = 40) |
Physical Frailty + Cognitive Impairment (n = 26) |
p Value |
|---|---|---|---|---|---|
| 1-y mortality (n = 20), n (%) | 9 (5.9) | 1 (1.1) | 5 (12.8) | 5 (20.0) | 0.002* |
| Survival rate at 1 y, % (95% CI) | 94.3 (89.3–97.0) | 98.9 (92.6–99.8) | 87.4 (72.4–94.6) | 80.3 (58.9–91.3) | — |
| 2-y mortality (n = 38), n (%) | 13 (9.9) | 6 (7.2) | 8 (22.9) | 11 (47.8) | <0.001* |
| Survival rate at 2 y, % (95% CI) | 91.4 (85.7–94.9) | 93.2 (85.4–96.9) | 79.4 (62.9–89.1) | 54.9 (33.3–72.1) | — |
| 3-y mortality (n = 50), n (%) | 19 (34.6) | 11 (26.2) | 9 (37.5) | 11 (64.7) | 0.048* |
| Survival rate at 3 y, % (95% CI) | 82.6 (72.5–89.3) | 86.3 (76.4–92.3) | 76.3 (59.3–87.0) | 54.9 (33.3–72.1) | — |
| Total death (n = 53), n (%) | 20 (11.9) | 12 (12.5) | 10 (25) | 11 (42.3) | <0.001* |
Statistically significant.
Composite frailty and overall survival
Long-term survival was collected and follow-up time of 3 years or more was available for 73.3% of the cohort. In the regression analyses age, albumin, and hemoglobin were entered as continuous variables and lower hemoglobin, lower albumin, and older age were correlated for risk of overall survival. In univariate analysis, the Physically Frail and Cognitively Impaired cohort (p < 0.001), age (p < 0.001), surgery for cancer (p = 0.002), Charlson Comorbidity Index (p = 0.022), albumin (p < 0.001), and hemoglobin (p = 0.011) were statistically significant predictors of overall survival.
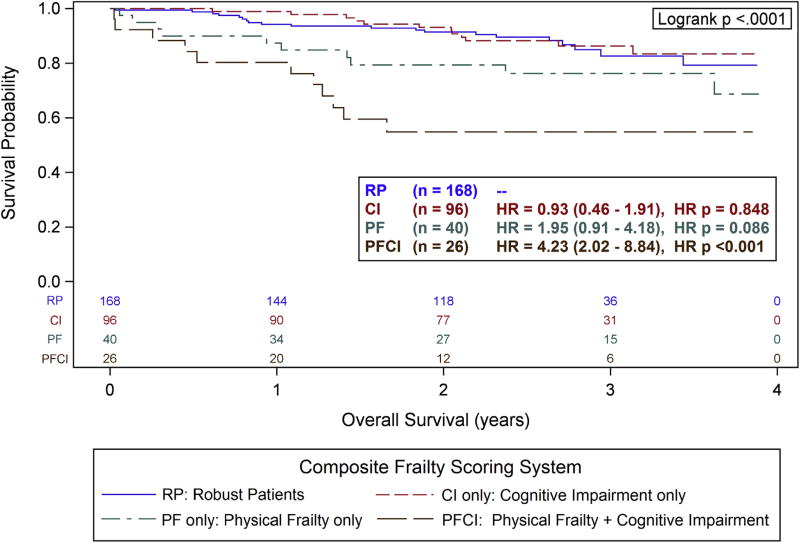
Kaplan-Meier curves (Fig. 3) reveal an overall significant difference in long-term survival (log rank p < 0.0001), driven mainly by the Physically Frail and Cognitively Impaired cohort. Unadjusted for confounders, the Physically Frail and Cognitively Impaired cohort had a 4.23 higher risk of dying (95% CI 2.02 to 8.84) when compared with the Robust patients (p < 0.001). Multivariable analyses revealed that the Physically Frail and Cognitively Impaired cohort had a 3.92 higher risk of death (95% CI 1.66 to 9.26) compared with the robust patients (p = 0.002). After adjusting for all significant variables to reduce confounding, the composite frailty cohorts were still significantly associated with overall survival (type 3, p = 0.010; Table 6).
Figure 3.
Kaplan-Meier plot of overall survival (years) stratified by the composite frailty scoring system. The number of patients at risk in each cohort is indicated at the bottom. Hazard ratio (HR) and corresponding 95% CI are from a Cox model adjusted for sex, Eastern Cooperative Oncology Group, estimated glomerular filtration rate, creatinine, hemoglobin, and race. The reference category was robust patients (RP cohort).
Table 6.
Univariate and Multivariable Analysis of Preoperative Variables as Predictors of Overall Survival (Months)
| Univariate analysis | Multivariable analysis* | |||
|---|---|---|---|---|
|
|
|
|||
| Preoperative variables | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Composite frailty score | 0.010†,‡ | |||
|
| ||||
| Robust | — | — | — | — |
|
| ||||
| Cognitive Impairment only | 0.93 (0.46–1.91) | 0.848 | 0.90 (0.38–2.13) | 0.811 |
|
| ||||
| Physical Frailty only | 1.95 (0.91–4.18) | 0.086 | 1.74 (0.75–4.04) | 0.195 |
|
| ||||
| Physical Frailty + Cognitive Impairment | 4.23 (2.02–8.84) | <0.001‡ | 3.92 (1.66–9.26) | 0.002‡ |
|
| ||||
| Age | 1.05 (1.02–1.07) | <0.001‡ | 1.04 (1.01–1.07) | 0.004‡ |
|
| ||||
| Sex (female vs male) | 1.27 (0.73–2.20) | 0.400 | — | — |
|
| ||||
| Race (non-white vs white) | 1.18 (0.66–2.12) | 0.583 | — | — |
|
| ||||
| Surgery for cancer (no vs yes) | 4.45 (1.77–11.20) | 0.002‡ | 2.50 (0.85–7.36) | 0.096 |
|
| ||||
| BMI, kg/m2 | 0.96 (0.92–1.01) | 0.112 | 0.976 (0.91–1.02) | 0.178 |
|
| ||||
| ASA score (1 to 2 vs 3 to 4) | 1.42 (0.71–2.83) | 0.317 | 2.32 (1.02–5.26) | 0.044‡ |
|
| ||||
| ECOG (0 to 1 vs 2 to 3) | 1.65 (0.51–5.29) | 0.400 | — | — |
|
| ||||
| CCI (0 to 1 vs 2) | 13.20 (1.82–95.60) | 0.011‡ | 5.24 (0.66–41.81) | 0.118 |
|
| ||||
| Albumin | 0.27 (0.17–0.41) | <0.001‡ | 0.26 (0.15–0.45) | <0.001‡ |
|
| ||||
| Hemoglobin | 0.85 (0.74–0.96) | 0.011‡ | — | — |
|
| ||||
| Creatinine | 0.99 (0.88–1.11) | 0.814 | — | — |
|
| ||||
| eGFR | 1.00 (1.00–1.00) | 0.113 | — | — |
|
| ||||
| Platelets | 1.00 (0.99–1.00) | 0.238 | 1.00 (0.99–1.00) | 0.034‡ |
Multivariable analysis was conducted with all independently significant preoperative variables.
Number of observations used was 253. Backwards selection with an α level of removal of 0.30 was used. The following variables were removed from the model: eGFR, creatinine, ECOG, sex, hemoglobin, and race.
Type 3 p value.
Statistically significant.
ASA, American Society of Anesthesiologists; CCI, Charlson Comorbidity Index; ECOG, Eastern Cooperative Oncology Group; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Composite frailty, surgery for cancer, and overall survival
There were 216 (65.5%) patients undergoing operations for cancer. In univariate analysis, cancer was a significant predictor of overall survival (p = 0.002); cancer patients had a 4.45 times higher risk of dying (95% CI 1.77 to 11.20) when compared with non-cancer patients. Given that cancer is a strong predictor of overall survival, we stratified our population into cancer patients and non-cancer patients for analysis. Log-rank tests were significant for both cancer patients (p = 0.0001) and non-cancer patients (p = 0.0467), respectively, demonstrating that the composite score had similar applicability in both groups. These findings were confirmed by multivariable analysis of overall survival for cancer patients (hazard ratio 2.85; 95% CI 1.16 to 6.98; HR p = 0.022).
DISCUSSION
Surgeons have found preoperative frailty to be a reliable objective method of quantifying a patient’s fitness for an operation.5,6,8,11,21 However, the definition of frailty used has most often been modeled after the Fried criteria and has therefore been limited primarily to the physical domain. Growing research demonstrates impaired preoperative cognitive impairment to be a predictor of poor postoperative outcomes.22,23 Robinson and colleagues found increased postoperative complications, longer hospital stays, higher rates of discharge institutionalization and higher 6-month mortality for geriatric surgery patients with baseline impaired cognition.11
Initially, the pathophysiologic mechanisms of physical frailty and cognitive impairment were studied separately. However, the interconnected relationship between a human’s physical and mental statuses has stimulated interest to create a more comprehensive concept of frailty.7,21,24–27 In our aging population, the accelerated risks of accumulating physical and mental deficits have been valuable and offer support for the concept of cognitive frailty.12,28 Data from the French Three-City study indicated that considering the cognition domain in the frailty phenotype improved the identification of frail persons at risk for adverse health outcomes in the French community-dwelling population aged 65 to 95 years during 4 years of follow-up.12
Considering the increased interest in a multidimensional assessment of frailty, our study integrates physical frailty and cognitive impairment to test a more robust risk assessment tool particularly for surgical patients. Although the CDT and frailty were not associated with each other on univariate analysis, they are both known in previous literature to have prognostic information related to postoperative outcomes.6,8,11,21–23 Therefore, we combined the cognitive and physical domains into a single score that we anticipated would outperform either alone. In our analysis, an abnormal CDT score combined with physical frailty carried an approximately 4-fold increase in the risk of mortality compared with the robust patients, which was confirmed on multivariable analysis.
This specific group of patients had a 42% rate of death during 4 years of follow-up, which was twice the risk of mortality when compared with patients who were frail without cognitive impairment. Additionally, the Physically Frail and Cognitively Impaired cohort had the most dramatic occurrence of deaths within 2 years when compared with the Robust patients (survival rate 80.4% at 1 year post operation to a 50% survival rate at 2 years post operation). When both cognition and physiologic reserve are impaired, patients are much less able to withstand the stress of an operation. In addition, the absence or presence of cognitive impairment in frail patients impacts how they met the greater demands of postoperative care. The results support this conclusion that combining the Fried frailty criteria and the CDT is a more effective predictor of overall survival than either measurement alone (Table 6). Yet, additional investigation is warranted to elucidate the underlying relationship and interdependencies between the physical and cognitive domains.
There are several unique aspects of our study. This is a large observational, prospective study of patients undergoing major operations from different surgical specialties. Previous studies only investigated the effect of physical frailty and cognitive impairment in one type of procedure or surgical discipline.21,24 However, our results indicate these findings apply to a large portion of surgical patients beyond these specific areas. Our study is unique for being the first to use the CDT as a surrogate for cognitive impairment and combine it with the Fried frailty criteria to create a 4-level composite frailty scoring system. In addition, the predictability of our composite score held true for both cancer patients and non-cancer patients, demonstrating that overall survival remains dependent on both the physical and cognitive reserve of a patient, regardless of the presence of a major comorbidity.
Also important to highlight is our application of the frailty definition to all surgical adult patients, not just the geriatric population. Many of the previous studies conducted on physical frailty and cognitive impairment focus only on adults aged 65 years and older.9,12,27,28 More than half (69.2%) of the physically frail and cognitively impaired patients in our cohort were aged 65 years or older. This is consistent with previous literature that the risk of cognitive and physical decline increases exponentially with age.13,25–30 However, the concept of the “young” frail patient is often neglected in many patient assessments, sometimes with dramatic negative consequences. As demonstrated by Revenig and colleagues,8 a substantial portion of patients aged 40 to 50 years who experienced postoperative complications were identified as intermediate frail. In our study, of the total 122 patients with cognitive impairment based on an abnormal CDT, half of our patients (50.8%) were younger than 65 years old. It is also worth noting that 8 of the 12 (66.7%) deceased Cognitively Impaired only patients and 3 of the 11 (27.3%) deceased Physically Frail and Cognitively Impaired patients were younger than 65 years of age. Nevertheless, of the total 264 patients classified as non-frail, 99 patients (37.5%) were in fact 65 years or older.
The concept of “young”’ frail or “old” non-frail patient is of significant interest and consistent with the idea that cognitive impairment, like physical frailty, is not merely a factor of chronological age. This highlights the importance of routinely measuring cognitive impairment in both the young and old adult populations during preoperative assessments, as well as before beginning other therapies that can result in adverse outcomes. Cognitive stimulation, which includes a variety of activities, such as word games, reminiscing, music, and puzzles aimed at providing mental exercises to maintain cognitive function, has been shown to benefit persons with mild to moderate dementia.31 It is unknown if prehabilitation interventions, including cognitive stimulation, could benefit a preoperative population with both frailty and cognitive impairment. Future investigative efforts should be promoted to evaluate whether preoperative intervention can positively affect a frail and cognitively impaired patient’s postoperative outcomes.
In our study, we implemented the CDT, which allowed for a simple, effective, and efficient screening tool for cognitive impairment in a fast-paced surgical clinic. Forti and colleagues32 also conducted a similar study using the combination of 3 different CDT protocols (Sunderland, Shulman, and the clock drawing interpretation scale) with the physical phenotype of frailty based on the Study of Osteoporotic Fractures index for the prediction of mortality in 766 dementia-free Italian community dwellers aged 65 years or older. In contrast to our findings, this study found that the Sunderland CDT can predict mortality risk independently of the physical phenotype of frailty, and combining these 2 measures does not improve their individual prognostic abilities. Although the differences in patient population, frailty scores, and use of different CDT protocols influence the contrasting results, it is worth noting that the association with mortality was not consistent across all CDT protocols.
Additionally, in contrast to previous findings,22,23 our assessment of cognitive impairment was not independently associated with poor postoperative outcomes. In fact, when incorporating the composite score among the non-frail population, Cognitive Impairment only patients in our study had similar outcomes after operations compared with the Robust patients. This is of clinical importance because these results suggest that a strong physical constitution can compensate for cognitive deficits or impairment. The presence of cognitive impairment alone in non-frail individuals might not be a sufficient outcomes predictor for surgical procedures.
There are several limitations to this study. First, there are several clock draw protocols and there is no universally used CDT.17–19,33–35 Additional research on diagnostic properties of clock draw protocols is important. Second, our study was conducted in an academic medical center in Atlanta, GA for a majority of Caucasian patients undergoing elective operations; therefore, results might not be generalizable to other study populations with different demographics. Third, we were limited in our ability to identify the cause of death for all patients using the CDC National Death Index. Fourth, our results might not apply to patients that cannot read, grip, or ambulate, as they were excluded from analysis. Although it is tempting to assume that these patients might be more physically and cognitively frail, we have no data to support this assumption. Fifth, this is a prospective, observational study and subject to selection bias and small sample sizes for events due to the somewhat short follow-up. Finally, we were limited in our ability to differentiate the outcomes between intermediate frail and frail patients, given the small number of frail patients in our study.
CONCLUSIONS
Our results highlight the predictive value of incorporating a combined assessment of physical frailty and cognitive impairment to risk stratify adults undergoing preoperative evaluation. Although most previous research has focused on physical frailty, our study suggests that combining physical frailty with cognitive impairment creates a valuable and more accurate predictor of postoperative survival, and independent assessment of cognitive impairment alone does not. In light of these findings, broadening the definition of cognitive frailty to be inclusive of not only the elderly population, but also the young adult population demonstrates that vulnerabilities in the physical and cognitive domains can limit recovery after the complex stressor of an operation. Moving forward, the utility of a combined frailty and cognitive assessment score to predict overall survival across surgical specialties has powerful potential to aid surgical risk stratification for preoperative decision making and possibly early intervention, as well as more accurate patient counseling.
Supplementary Material
Acknowledgments
The efforts of Martha Henderson to assist in obtaining clinical data for this study are gratefully acknowledged.
Support: Research reported in this publication was supported in part by the biostatistics and bioinformatics of Winship Cancer Institute of Emory University and NIH/National Cancer Institute under award number P30CA138292. Dr Paul Garcia’s research efforts are supported by funding by the Department of Veterans Affairs (CDA BX001677) and the James S McDonnell Foundation Grant #220023046.
Footnotes
Disclosure Information: Authors have nothing to disclose. Timothy J Eberlein, Editor-in-Chief, has nothing to disclose.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions
Study conception and design: Vaughan, Johnson, García, Ogan, Master
Acquisition of data: Makhani, Kim, Li, Revenig
Analysis and interpretation of data: Makhani, Kim, Liu, Ye
Drafting of manuscript: Makhani, Kim
Critical revision: Makhani, Kim, Liu, Ye, Li, Revenig, Vaughan, Johnson, García, Ogan, Master
References
- 1.Sakland M. Grading of patients for surgical procedures. Anesthesiology. 1941;2:281–284. [Google Scholar]
- 2.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–656. [PubMed] [Google Scholar]
- 3.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Li JL, Henderson MA, Revenig LM, et al. Frailty and one-year mortality in major intra-abdominal operations. J Surg Res. 2016;203:507–512.e1. doi: 10.1016/j.jss.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Revenig LM, Canter DJ, Kim S, et al. Report of a simplified frailty score predictive of short-term postoperative morbidity and mortality. J Am Coll Surg. 2015;220:904–911.e1. doi: 10.1016/j.jamcollsurg.2015.01.053. [DOI] [PubMed] [Google Scholar]
- 7.Jha SR, Hannu MK, Chang S, et al. The prevalence and prognostic significance of frailty in patients with advanced heart failure referred for heart transplantation. Transplantation. 2016;100:429–436. doi: 10.1097/TP.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 8.Revenig LM, Canter DJ, Taylor MD, et al. Too frail for surgery? Initial results of a large multidisciplinary prospective study examining preoperative variables predictive of poor surgical outcomes. J Am Coll Surg. 2013;217:665–670.e1. doi: 10.1016/j.jamcollsurg.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Neupane I, Arora RC, Rudolph JL. Cardiac surgery as a stressor and the response of the vulnerable older adult. Exp Gerontol. 2017;87(Pt B):168–174. doi: 10.1016/j.exger.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250:449–455. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 12.Ávila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57:453–461. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 13.Kelaiditi E, Cesari M, Canevelli M, et al. Cognitive frailty: rational and definition from an (IANA/IAGG) international consensus group. J Nutr Health Aging. 2013;17:726–734. doi: 10.1007/s12603-013-0367-2. [DOI] [PubMed] [Google Scholar]
- 14.Steenland NK, Auman CM, Patel PM, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. J Alzheimers Dis. 2008;15:419–427. doi: 10.3233/jad-2008-15308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lessig MC, Scanlan JM, Nazemi H, Borson S. Time that tells: critical clock-drawing errors for dementia screening. Int Psychogeriatr. 2008;20:459–470. doi: 10.1017/S1041610207006035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters R, Pinto EM. Predictive value of the clock drawing test. Dement Geriatr Cogn Disord. 2008;26:351–355. doi: 10.1159/000162261. [DOI] [PubMed] [Google Scholar]
- 17.Mendez MF, Ala T, Underwood KL. Development of scoring criteria for the clock drawing task in Alzheimer’s disease. J Am Geriatr Soc. 1992;40:1095–1099. doi: 10.1111/j.1532-5415.1992.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 18.Reichenfeld HF, Wells GA. Clock drawing: a neuropsychological analysis. J Psychiatry Neurosci. 1995;20:155. [Google Scholar]
- 19.Shulman KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry. 2000;15:548–561. doi: 10.1002/1099-1166(200006)15:6<548::aid-gps242>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Rubenstein LZ, Harker JO, Salvà A, et al. Screening for undernutrition in geriatric practice developing the short-form mininutritional assessment (MNA-SF) J Gerontol A Biol Sci Med Sci. 2001;56:M366–M372. doi: 10.1093/gerona/56.6.m366. [DOI] [PubMed] [Google Scholar]
- 21.Robinson TN, Wu DS, Pointer L, et al. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg. 2013;206:544–550. doi: 10.1016/j.amjsurg.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrington MB, Kraft M, Grande LJ, Rudolph JL. Preoperative cognitive status is independently associated with discharge location after cardiac surgery. Am J Crit Care. 2011;20:129. doi: 10.4037/ajcc2011275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson TN, Wu DS, Pointer LF, et al. Preoperative cognitive dysfunction is related to adverse postoperative outcomes in the elderly. J Am Coll Surg. 2012;215:12–17. doi: 10.1016/j.jamcollsurg.2012.02.007. discussion 17–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jha SR, Hannu MK, Gore K, et al. Cognitive impairment improves the predictive validity of physical frailty for mortality in patients with advanced heart failure referred for heart transplantation. J Heart Lung Transplant. 2016;35:1092–1100. doi: 10.1016/j.healun.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Aguilar-Navarro SG, Mimenza-Alvarado AJ, Anaya-Escamilla A, Gutiérrez-Robledo LM. Frailty and vascular cognitive impairment: mechanisms behind the link. Rev Invest Clin. 2016;68:25. [PubMed] [Google Scholar]
- 26.Avila-Funes JA, Pelletier A, Meillon C, et al. Vascular cerebral damage in frail older adults: the AMImage study. J Gerontol A Biol Sci Med Sci. 2017;72:971–977. doi: 10.1093/gerona/glw347. [DOI] [PubMed] [Google Scholar]
- 27.Boyle PA, Buchman AS, Wilson RS, et al. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58:248–255. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griebling TL. Re: accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Urol. 2012;188:1213–1214. doi: 10.1016/j.juro.2012.06.107. [DOI] [PubMed] [Google Scholar]
- 29.Panza F, Seripa D, Solfrizzi V, et al. Targeting cognitive frailty: clinical and neurobiological roadmap for a single complex phenotype. J Alzheimers Dis. 2015;47:793–813. doi: 10.3233/JAD-150358. [DOI] [PubMed] [Google Scholar]
- 30.Searle SD, Rockwood K. Frailty and the risk of cognitive impairment. Alzheimers Res Ther. 2015;7:54. doi: 10.1186/s13195-015-0140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012 Feb;2:1–81. doi: 10.1002/14651858.CD005562.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Forti P, Maioli F, Lega MV, et al. Combination of the clock drawing test with the physical phenotype of frailty for the prediction of mortality and other adverse outcomes in older community dwellers without dementia. Gerontology. 2014;60:204–211. doi: 10.1159/000356701. [DOI] [PubMed] [Google Scholar]
- 33.Amodeo S, Mainland BJ, Herrmann N, Shulman KI. The times they are a-changin’ clock drawing and prediction of dementia. J Geriatr Psychiatry Neurol. 2015;28:145–155. doi: 10.1177/0891988714554709. [DOI] [PubMed] [Google Scholar]
- 34.Caffarra P, Gardini S, Zonato F, et al. Italian norms for the Freedman version of the Clock Drawing Test. J Clin Exp Neuropsychol. 2011;33:982–988. doi: 10.1080/13803395.2011.589373. [DOI] [PubMed] [Google Scholar]
- 35.Mainland BJ, Amodeo S, Shulman KI. Multiple clock drawing scoring systems: simpler is better. Int J Geriatr Psychiatry. 2014;29:127–136. doi: 10.1002/gps.3992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.