Figure 7.
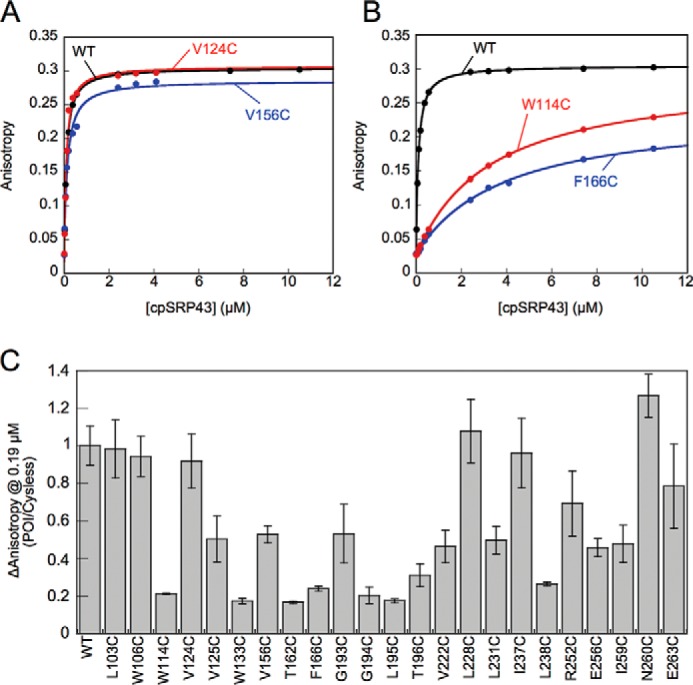
Characterization of the interaction of mutant cpSRP43s with the L18 motif. A and B, representative equilibrium titrations for the binding of WT and mutant cpSRP43s to HiLyte-Fluor 488-labeled L11. Representative data for cpSRP43 mutants that can bind L11 with high affinity are shown in A, and those for mutants exhibiting weakened L11 binding are shown in B. C, summary of the cpSRP43-induced changes in the fluorescence anisotropy of L11 at 0.19 μm, which is subsaturating for binding of Cys-less cpSRP43 to L11. The data for all mutants are normalized to that of Cys-less cpSRP43 (denoted as WT). All data are reported as mean ± S.E., with n ≥ 2.
