Figure 8.
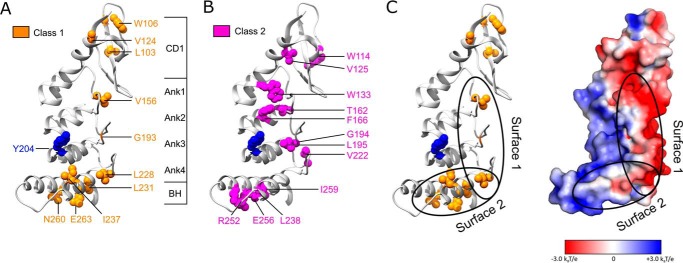
Mapping two classes of cpSRP43 mutants onto the crystal structure of the cpSRP43 SBD (Protein Data Bank code 3dep). A, residues whose mutations led to defective chaperone activity for LHCP but did not disrupt L18 binding are categorized as Class I and colored in orange. B, residues whose mutations disrupted both cpSRP43's chaperone activity and its interaction with the L18 motif are categorized as Class II and colored in magenta. C, a putative model for the interaction surfaces of cpSRP43 with LHCP, with Tyr-204 (blue) interacting with the L18 sequence, and the hydrophobic surfaces formed by Ank4, BH, and the β-hairpins along the ankyrin repeat motifs involved in protection of the TMDs of LHCP. The electrostatic surface potential of the cpSRP43 SBD was generated using Adaptive Poisson-Boltzmann Solver (52) and visualized in PyMOL.