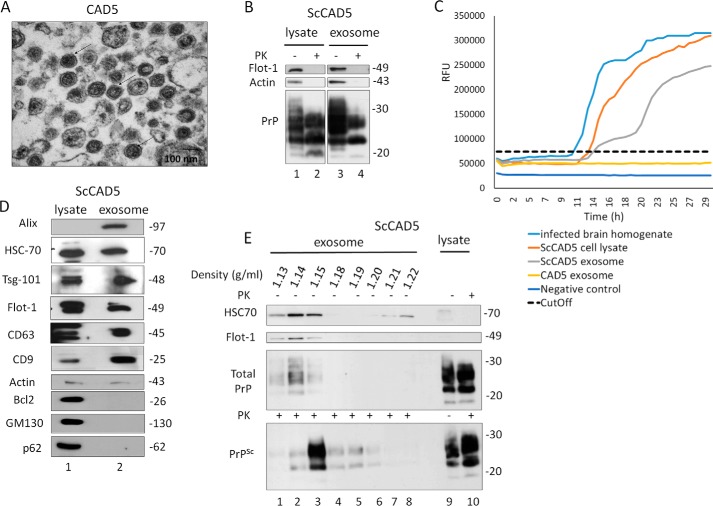
Figure 1.
Characterization of exosomes isolated from CAD5/ScCAD5 neuronal cells. A, representative TEM of exosomes isolated from CAD5 culture medium reveals a homogenous population of vesicles of 100 nm in diameter characteristic for exosomes (some denoted by black arrows). Scale bar, 100 nm. B, immunoblot of ScCAD5 cell lysate and exosome preparations probed for total PrP (−PK) and PrPSc (+PK) (anti-PrP mAb 4H11). Actin was used as loading control. Flotillin-1 (Flot-1) was used as an exosome marker. C, RT-QuIC of CAD5 exosome, ScCAD5 exosome, ScCAD5 cell lysate, 10% brain homogenate from terminally prion-sick mice (22L) or left unseeded (negative control). The average increase of thioflavin-T fluorescence of replicate wells is plotted as a function of time. The y axis represents RFU, and the x axis represents time (h). D, immunoblot analysis of ScCAD5 cell lysate and exosomes isolated from ScCAD5 culture medium. Exosome preparation is positive for exosome markers Alix, HSC70, Tsg-101, flotillin-1, CD63, and CD9 and negative for mitochondrial marker Bcl2, Golgi marker GM130, and nuclear marker nucleoporin p62. Actin was used loading control. E, ScCAD5 exosome pellet loaded on the top of a continuous sucrose gradient and ultracentrifuged. Fractions were analyzed by Western blotting and probed for HSC70, flotillin-1, and mAb 4H11 to detect PrP/PrPSc. Lanes 9 and 10 represent cell lysate before and after PK digestion, respectively.