Figure 2.
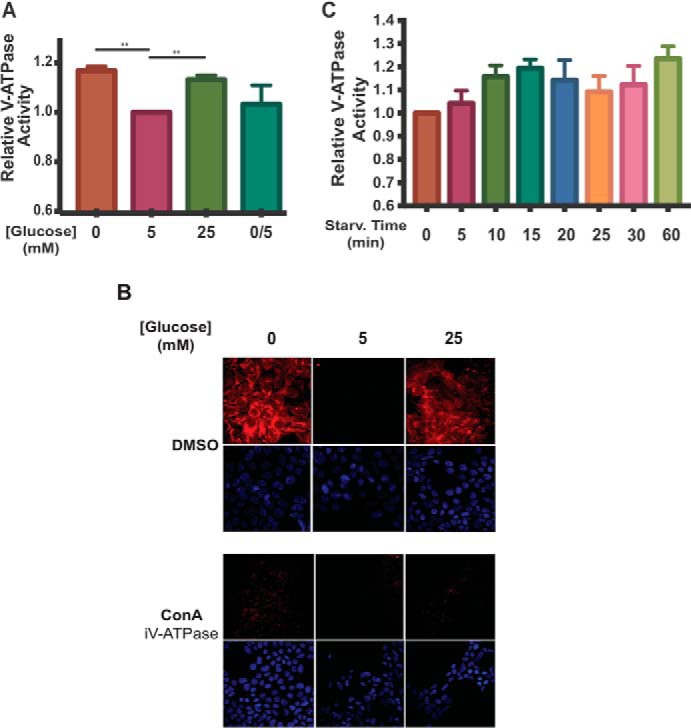
Incubation of HEK293T cells at low or high glucose increases V-ATPase-dependent acidification of lysosomes. A, HEK293T cells were incubated with FITC-dextran to load lysosomes by fluid-phase endocytosis as described under “Experimental procedures.” Cells were then maintained in serum-free DMEM containing 5 mm glucose for ∼6 h and then treated with serum-free DMEM containing 0 mm glucose (10 min.), 5 mm glucose (1 h), 25 mm glucose (1 h), or 0 mm glucose (10 min.) followed by 5 mm glucose (10 min.). Following treatment, lysosomes were isolated from cells as described under “Experimental procedures.” Fluorescence intensity at 520 nm (excitation at 490 nm) was measured as a function of time following addition of Mg-ATP. The rate V-ATPase-dependent proton transport activity for each condition was determined by performing a linear regression analysis on the initial rate of concanamycin A-sensitive fluorescence quenching (where present, concanamycin A was added at 1 μm). Rates of fluorescence quenching from independent trials were normalized to the baseline value observed for lysosomes isolated from cells maintained at 5 mm glucose (defined as 1.0). The average lysosomal V-ATPase activity for lysosomes from cells treated with 0 mm glucose was 1.17 ± 0.02 (p < 0.01, n = 20), and for cells treated with 25 mm glucose the average activity was 1.13 ± 0.02 (p < 0.01, n = 20). There was no significant difference in activity between lysosomes from cells treated with 5 mm glucose compared with lysosomes from cells treated with 0 mm glucose followed by 5 mm glucose (0/5). The error bars represent the mean ± S.E. The asterisks indicate statistically significant differences at the indicated p values. B, HEK293T cells were treated with glucose as described in A. Concanamycin A (ConA) at 5 μm or DMSO was added to the cells 1 h before collection. 10 min prior to cell collection, cells were incubated with LysoTracker to stain for acidic cellular compartments and then fixed as described under “Experimental procedures.” Staining was detected by confocal fluorescence microscopy (red, LysoTracker; blue, DAPI), and representative images are shown, n = 2. C, FITC-dextran loaded HEK293T cells were maintained in serum-free DMEM containing 5 mm glucose for ∼6 h and then treated with 0 mm glucose for the indicated amounts of time. Rates of fluorescence quenching were measured as in A. n = 3.
