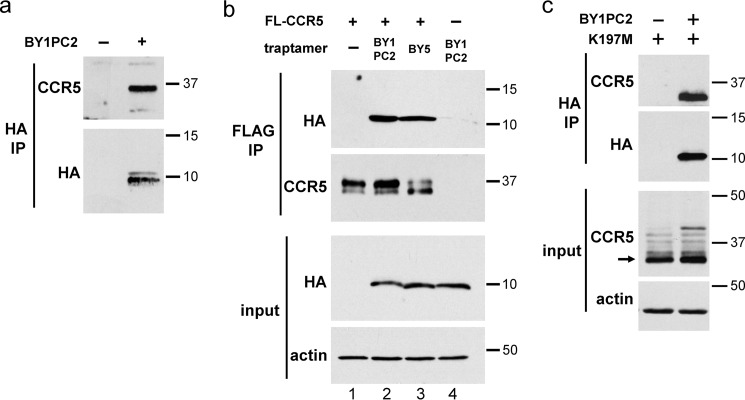
Figure 6.
Class 1 traptamers form a stable complex with CCR5 and the K197M CCR5 mutant. a, BaF3 cells expressing FLAG-tagged CCR5 (FL-CCR5) in the absence (−) or presence (+) of BY1PC2 were treated with 25 μm chloroquine for 24 h and lysed, and extracts were immunoprecipitated (IP) with anti-FLAG or anti-HA antibody, as indicated. Immunoprecipitates were subjected to SDS-PAGE followed by Western blotting using a anti-HA antibody. Similar amounts of FL-CCR5 in both extracts are documented in b, second panel, lanes 1 and 2. b, parental BaF3 cells (lane 4) and BaF3 cells expressing FLAG-tagged CCR5 in the presence or absence of BY1PC2 or BY5, as indicated, were treated with 25 μm chloroquine for 24 h and lysed. Extracts were immunoprecipitated with anti-FLAG antibody and subjected to SDS-PAGE followed by Western blotting with antibody recognizing the traptamer (anti-HA) or CCR5, as indicated. Filters of input extracts were probed with anti-HA or with anti-actin antibody, as indicated. c, detergent extracts from BaF3 cells expressing the CCR5/K197M mutant in the presence or absence of BY1PC2 were immunoprecipitated with an anti-HA antibody, subjected to SDS-PAGE, and Western blotted with an anti-HA antibody or with an anti-CCR5 antibody. Filters of input extracts were probed with an anti-CCR5 antibody or with anti-actin antibody as a loading control. Numbers to the right in all panels indicate the size in kilodaltons of molecular mass standards.