Figure 8.
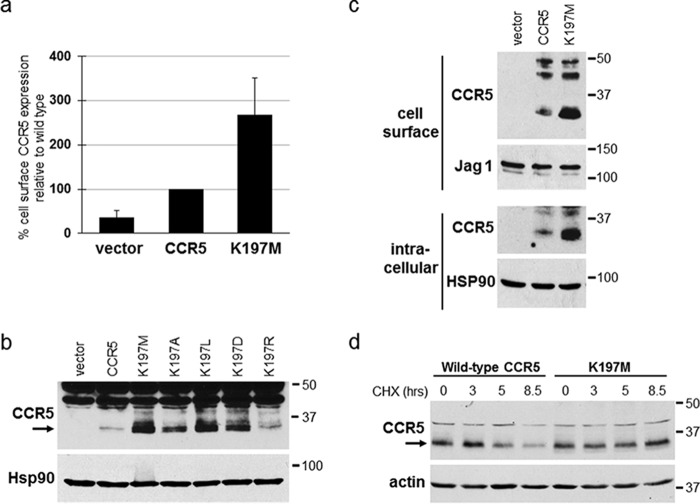
Lysine 197 reduces the stability of CCR5 in the absence of traptamers. a, BaF3 cells expressing empty (MSCVn) vector or BaF3 cells expressing WT or K197M CCR5 were established in parallel, and cell-surface expression of each receptor was analyzed by flow cytometry of nonpermeabilized cells by using 2D7 anti-CCR5 antibody. The geometric mean fluorescence intensity for each sample was determined and normalized to cells expressing WT CCR5. Average and S.D. (error bars) for three experiments are shown. b, BaF3 cells expressing the empty vector, WT CCR5, or the indicated CCR5 point mutant were established in parallel. Detergent lysates were subjected to SDS-PAGE and Western blotting for CCR5 or Hsp90 as a loading control. c, the cells studied in a were subjected to cell-surface biotinylation, and biotinylated cell-surface proteins were recovered from cell extracts with streptavidin beads. The pellet (cell-surface) and supernatant (intracellular) fractions were subjected to SDS-PAGE and immunoblotted using an anti-CCR5 antibody. Samples were also probed for Jag1 or Hsp90 as loading controls. d, BaF3 cells expressing WT CCR5 or CCR5/K197M were treated with cyclohexamide (CHX), and at the indicated times, a portion of the culture was pelleted and lysed. Cell lysates were electrophoresed and immunoblotted for CCR5. The filters were stripped and reprobed for actin as a loading control. Numbers to the right of b, c, and d indicate the size in kilodaltons of molecular mass standards.