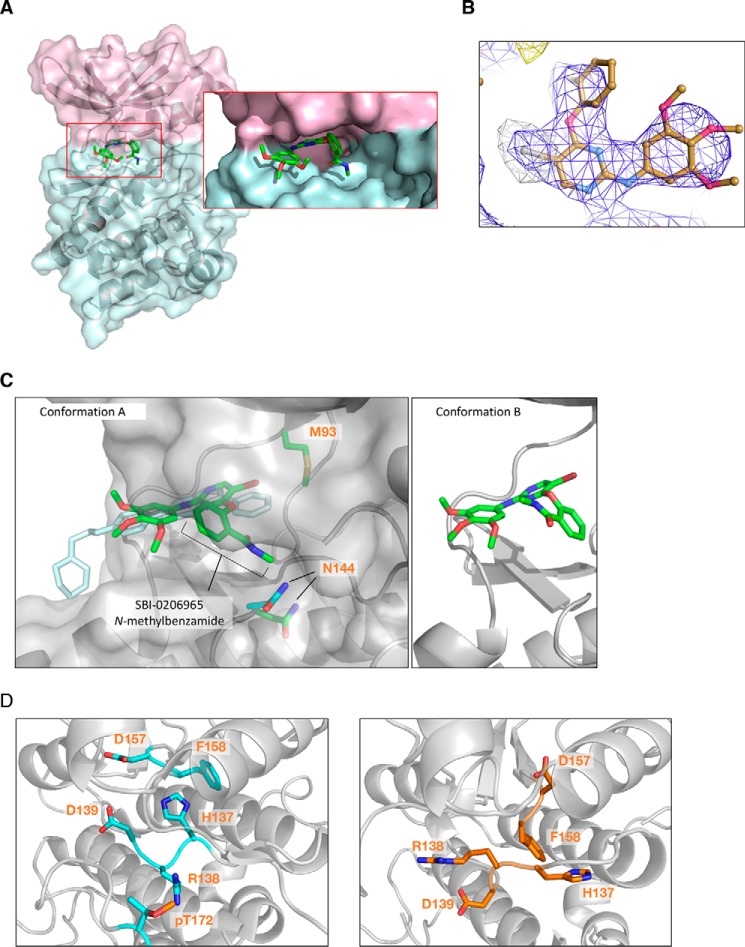
Figure 4.
A, crystal structure of α2 kinase domain (residues 6–278) (T174D) complexed to SBI-0206965 in conformation A. Pink, N-lobe; blue, C-lobe. B, anomalous difference map at 3.0 Å of SBI-0206965 bromine contoured to 5.0 σ collected at the bromine edge (13.6 keV). The anomalous bromine peak (white mesh) strongly supports the placement of SBI-0206965 within the electron density (blue mesh). C, left, overlapping SBI-0206965 (green, conformation A) and compound C (cyan) binding sites. Surface represents SBI-0206965–bound α2. Gatekeeper residue α2-Met-93 and catalytic loop residue Asn-144 are shown (green, SBI-0206965 complex; cyan, compound C complex). Right, SBI-0206965 in conformation B. D, comparison of HRD motifs in the active α2 kinase conformation (PDB 4ZHX) (left) and α2 kinase domain complexed to SBI-0206965 (right).